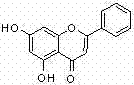
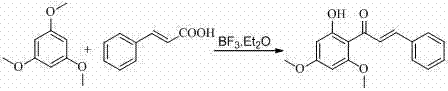Method of preparing chrysin
A technique for chrysin and trimethoxybenzene, applied in the field of preparing chrysin, can solve the problems of high price, low synthesis total yield and efficiency, long route, etc., and achieves improved synthesis yield and efficiency, high industrial application prospect , the effect of simplifying the synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0019] The method for preparing chrysin disclosed in the present invention can be easily implemented by those skilled in the art by referring to the content of the present application. In particular, it should be pointed out that all similar replacements and modifications will be obvious to those skilled in the art. The method of the present invention is described through the following examples, and relevant personnel can obviously do it without departing from the content and spirit of the present invention. The inventive technique is implemented for the methods described herein. These similar replacements and modifications should also be placed within the claims of the patent of this application.
[0020] The concrete implementation content of this preparation chrysin is as follows:
Embodiment 1
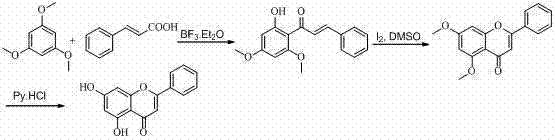
[0022] (1) Synthesis of 2'-hydroxy-4',6'-dimethoxychalcone
[0023]
[0024] Weigh 4.2g of 1,3,5-trimethoxybenzene (0.025mol) and 3.7g of cinnamic acid (0.025mol) into a dry round bottom flask, then add 15.0mL of boron trifluoride ethyl ether, and condense at 80°C with a drying tube reflow. After reacting for 3 hours, the heating was stopped to room temperature, and red needle crystals were precipitated, and the crystals were filtered out. The crystals were added to 100 mL of ethanol aqueous solution and heated to reflux for 2.5 hours to obtain an orange-yellow clear liquid, which was decolorized and filtered by adding activated carbon. After cooling, the yellow solid was washed out, filtered, washed and dried to obtain 5.35g of 2'-hydroxy-4',6'-dimethoxychalcone, with a yield of 75.4%.
[0025] Structural parameters 1 H NMR (400 MHz, DMSO-d 6 ), δ: 3.83 (s, 3H, OCH 3 -4’), 3.91 (s, 3H, OCH 3 -6'), 6.14 (d, J = 1.6 Hz, 1H, H-3'), 6.17 (d, J = 1.6 Hz, 1H, H-5'), 7.45–...
Embodiment 2
[0035] (1) Synthesis of 2'-hydroxy-4',6'-dimethoxychalcone
[0036]
[0037] Weigh 4.2g of 1,3,5-trimethoxybenzene (0.025mol) and 5.5g of cinnamic acid (0.0375mol) into a dry round bottom flask, then add 30.0mL of boron trifluoride ether, and condense at 100°C with a drying tube reflow. After reacting for 4 hours, the heating was stopped to room temperature, and red needle-like crystals were precipitated, and the crystals were filtered out. The crystals were added to 100 mL of ethanol aqueous solution and heated to reflux for 2.5 hours to obtain an orange-yellow clear liquid, which was decolorized and filtered by adding activated carbon. After cooling, the yellow solid was washed out, filtered, washed and dried to obtain 5.79g of 2'-hydroxy-4',6'-dimethoxychalcone with a yield of 81.6%.
[0038] (2) Synthesis of 5,7-dimethoxyflavone
[0039]
[0040] Weigh 5.7g (0.02 mol) of 2'-hydroxy-4',6'-dimethoxychalcone, add 30 mL DMSO to dissolve completely, add 0.17g elemental...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com