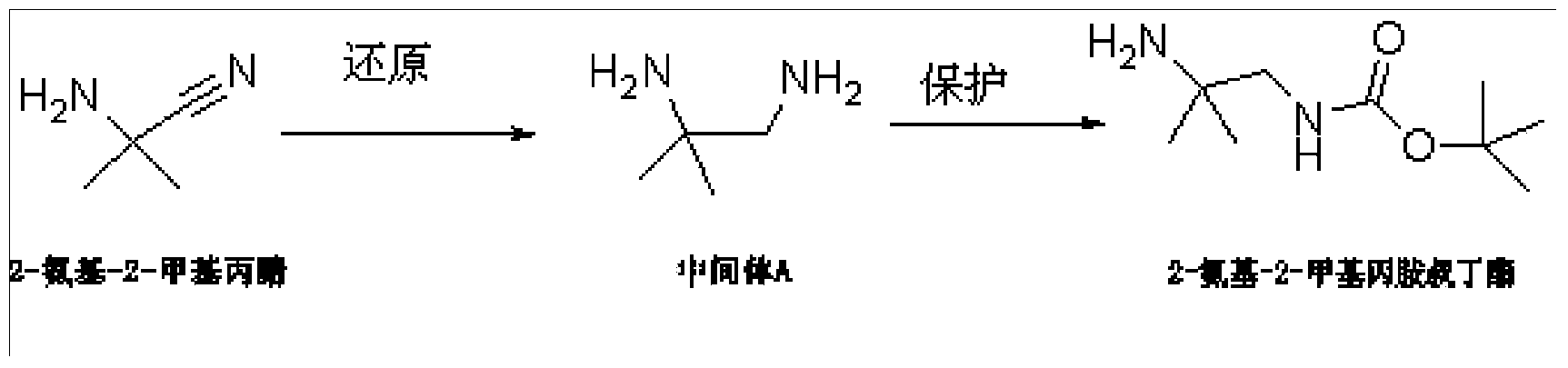
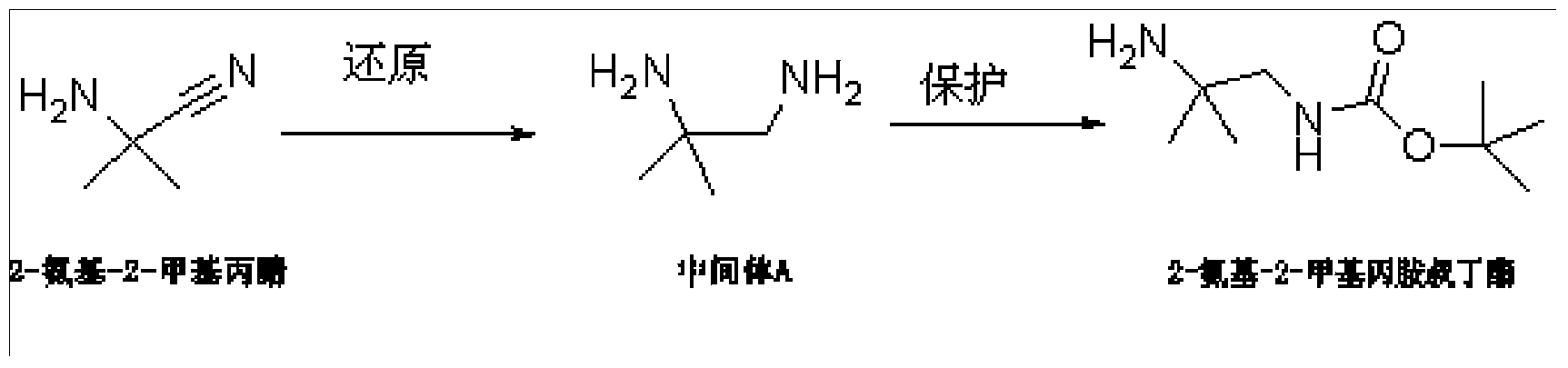
Synthesis method of anagliptin intermediate 2-amino-2-methylpropylamine tert-butyl ester
A technique for the synthesis of tert-butyl methylpropylamine, which is applied in the field of medicine, can solve the problems of expensive raw materials, time-consuming, difficult to obtain, etc., and achieve the effects of short synthesis route, mild reaction conditions, and little pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: the synthetic method of alalogliptin intermediate 2-amino-2-methylpropylamine tert-butyl ester, specifically comprises the following steps:
[0031] S1. In a three-necked flask, suspend 2-amino-2-methylpropionitrile hydrochloride in toluene, slowly add aluminum hydride dropwise within 30 minutes, raise the temperature to 100°C and stir for 1 hour after the addition, and wait for After the reaction is completed, cool to room temperature, slowly add distilled water dropwise to decompose excess aluminum hydride, adjust the pH to 8 with sodium hydroxide solution, add ethyl acetate, separate the liquids, and concentrate the combined organic phase after drying over anhydrous sodium sulfate , intermediate A is obtained; wherein, the weight ratio of 2-amino-2-methylpropionitrile, toluene, aluminum hydride is 1:1:0.05;
[0032] S2. In a three-necked flask, dissolve intermediate A and triethylamine in toluene, add tert-butoxycarbonyl anhydride, stir at room temperat...
Embodiment 2
[0033] Embodiment 2: the synthetic method of alalogliptin intermediate 2-amino-2-methylpropylamine tert-butyl ester, specifically comprises the following steps:
[0034] S1. In a three-necked flask, suspend 2-amino-2-methylpropionitrile hydrochloride in tetrahydrofuran, slowly add borane dropwise within 30 minutes, raise the temperature to 25°C and stir for 100 hours after the addition, and wait for After the reaction is completed, cool to room temperature, slowly add distilled water dropwise to decompose excess borane, adjust the pH to 9 with sodium hydroxide solution, add ethyl acetate, separate the layers, and concentrate the combined organic phase after drying over anhydrous sodium sulfate. Prepare intermediate A; Wherein, the weight ratio of 2-amino-2-methylpropionitrile, tetrahydrofuran, borane is 1:20:4;
[0035] S2. In a three-necked flask, dissolve intermediate A and triethylamine in tetrahydrofuran, then add tert-butoxycarbonyl chloride, stir at room temperature afte...
Embodiment 3
[0036] Embodiment 3: the synthetic method of alalogliptin intermediate 2-amino-2-methylpropylamine tert-butyl ester, specifically comprises the following steps:
[0037] S1. In the reaction kettle, suspend Raney nickel and 2-amino-2-methylpropionitrile hydrochloride in methanol, and replace the air in the reaction kettle with hydrogen for 3 times. After completion, pressurize and heat up to 40°C and stir 24h, then cooled to room temperature, filtered, and the organic phase was concentrated to obtain intermediate A; wherein, the weight ratio of 2-amino-2-methylpropionitrile, dichloromethane, and Raney nickel was 1:8:1.2;
[0038] S2. In a three-necked flask, dissolve intermediate A and diisopropylamine in dichloromethane, add tert-butoxycarbonylphenyl ester, stir at room temperature after the addition, then raise the temperature to 45°C and stir for 8 hours, and cool down after the reaction is complete to room temperature, adjust the pH to 9 with sodium hydroxide solution, extr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com