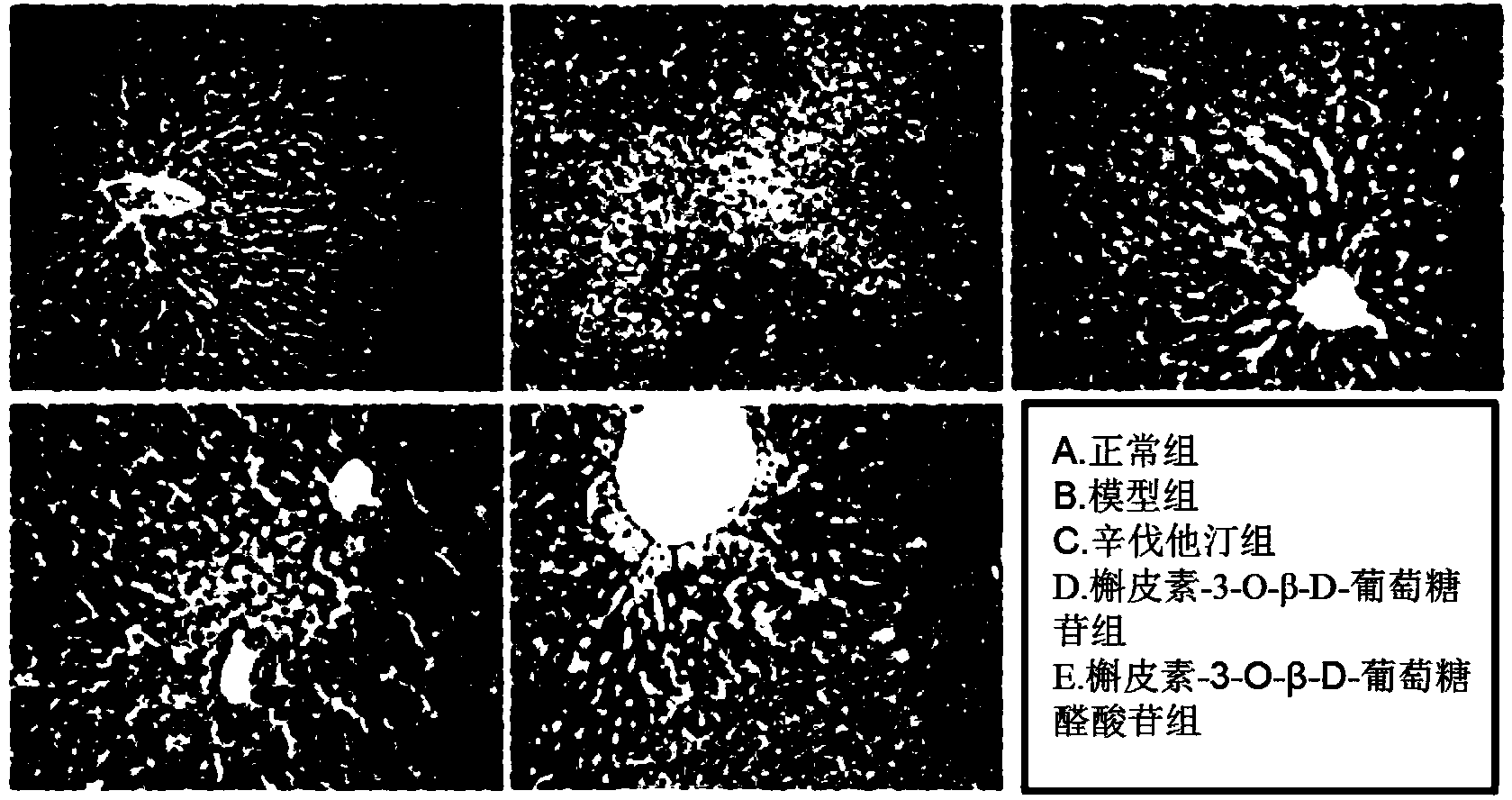
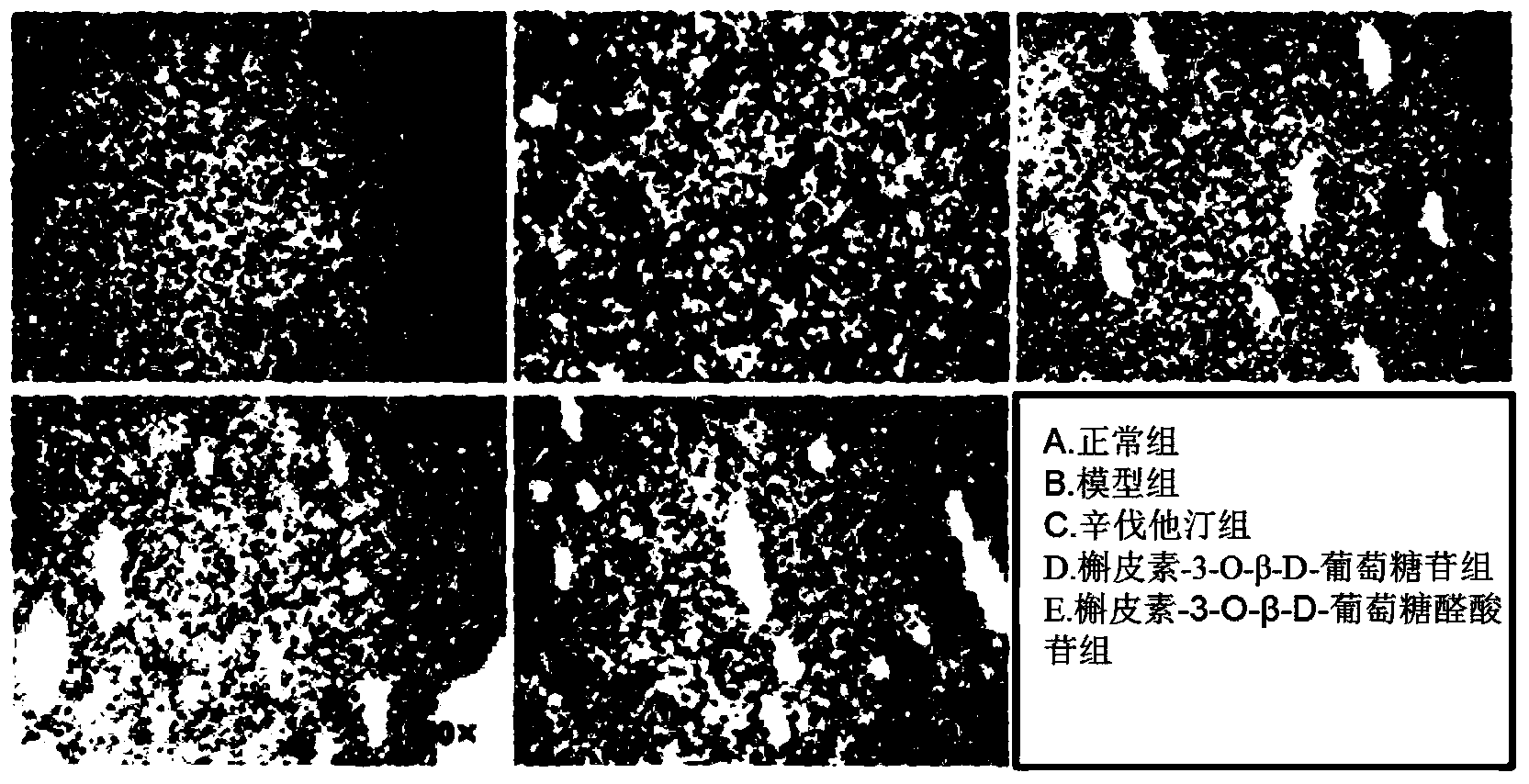
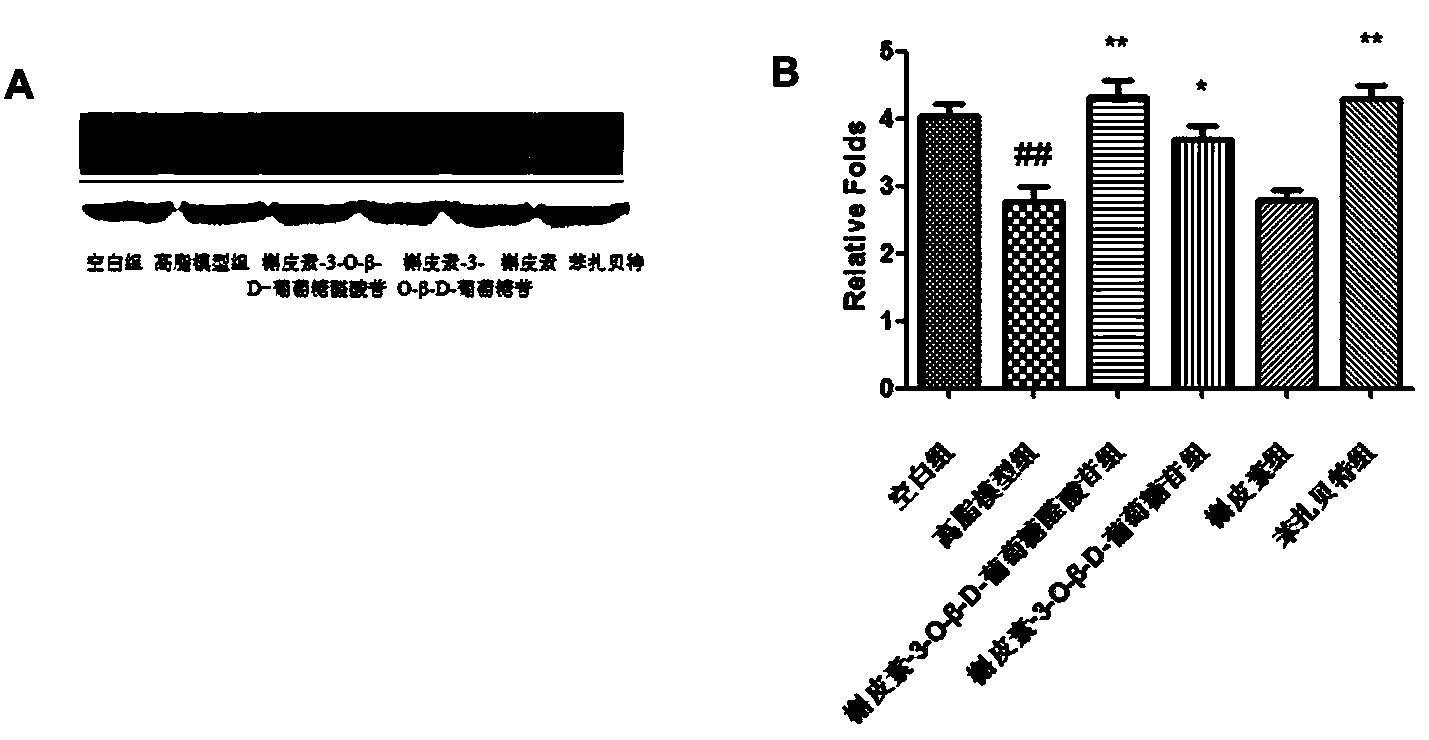
Application of quercetin-O-glucoside derivative to treatment of lipid metabolism disorders
A compound and solvate technology, applied in the field of medicine, can solve the problems of complex pathogenesis and difficult treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Synthesis of Quercetin-3-O-β-D-glucoside (Compound 2)
[0067] Put 50 g (150 mmol) of quercetin, 50 mL (256 mmol) of diphenylmethylene chloride, and 100 mL of diethylene glycol dimethyl ether into a 200 mL single-necked flask, and heat to reflux for 10 minutes. The solvent was distilled off under reduced pressure to obtain a yellow syrup, which was purified by silica gel column chromatography to obtain a light yellow solid (35.67g, 46.32%). 23.3g (50mmol) of the compound obtained in the previous step was followed by 13.8g (100mmol) of potassium carbonate Dissolve in tetrahydrofuran solution (100mL), slowly add 19.85g (50mmol) of acetyl bromide-α-D-glucose to it under stirring, control the temperature of the reaction solution at 0-5°C, and continue to stir and react at this temperature for 8 Hour. The reaction solution was diluted with 40 mL of dichloromethane, filtered to remove the remaining potassium carbonate, the filtrate was distilled, the organic layer was dried ...
Embodiment 2
[0070] Synthesis of 3',4'-Dihydroxymethylquercetin-3-O-β-D-glucoside (Compound 3)
[0071] The first two steps are the same as in Example 1. The obtained light yellow syrup (6.18g, 10mmol), bromomethanol (1.09g, 10mmol), and potassium carbonate (1.38g, 10mmol) are successively dissolved in 50ml of tetrahydrofuran solution, and the heating bar Added and refluxed for 7 hours, and then purified by silica gel column chromatography to obtain a light yellow solid, to which was added 15 mL of sodium methoxide solution, reacted at room temperature for 3 hours, and separated by polyamide column chromatography (chloroform:methanol=7:1). A light yellow solid (3.23 g, 67.2%) was obtained, mp 231-235°C.
[0072] 1 H-NMR (400MHz, DMSO-d 6 ): δ3.34–3.67(m,6H),4.27-4.31(m,4H),5.25(d,J=7.6Hz,1H),6.16(d,J=1.6Hz,1H),6.38(d, J=2.0Hz, 1H), 6.54(s, 2H), 6.56(d, J=8.4Hz, 1H), 7.46(dd, J=8.4, 2.0Hz, 1H), 7.75(d, J=2.0Hz, 1H).MS(m / z):525.3[M+H] + .
Embodiment 3
[0074] Synthesis of Quercetin-3-O-β-D-glucuronide (Compound 4)
[0075] The preparation process is the same as in Example 1, except that the acetyl bromide-α-D-glucose used in the second step is replaced by acetyl bromide-α-D-glucuronide methyl ester, the reaction yield is 46.7%, mp 245~248°C.
[0076] 1 H-NMR (400MHz, DMSO-d 6 ): δ3.30–3.60(m,6H),5.15(d,J=7.6Hz,1H),6.10(d,J=1.6Hz,1H),6.30(d,J=2.0Hz,1H),6.71 (d,J=8.4Hz,1H),7.49(dd,J=8.4,2.0Hz,1H),8.27(d,J=2.4Hz,1H).MS(m / z):479.1[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com