Composition and preparation method of high-activity defluorinating agent for nitrogen trifluoride anhydrous decomposition reaction
A technology of decomposition reaction and nitrogen trifluoride, which is applied in separation methods, chemical instruments and methods, and separation of dispersed particles, can solve problems such as high energy consumption, complicated operation process, and strong corrosion of reactors, and achieve high reactivity, The effect of preparation process parameter control and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
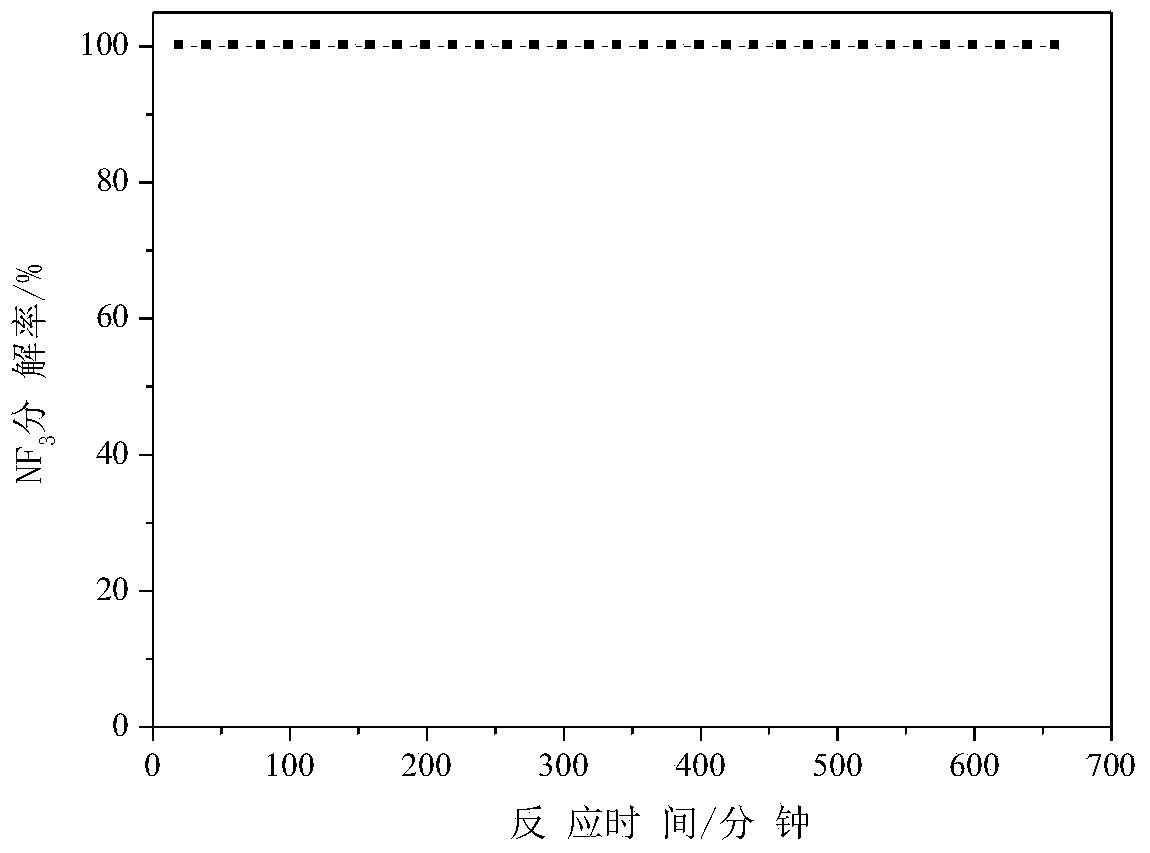
[0034] Taking by weighing 0.651 gram of mass concentration is 50% manganese nitrate (molecular formula is Mn(NO 3 ) 2 , the molecular formula of manganese nitrate in the following examples is the same) solution, diluted with water to 8.5 ml, impregnated with 5 grams of alumina particles for 24 hours, dried at 100°C for 6 hours, and roasted at 600°C for 3 hours to obtain manganese oxide-alumina Fluorine agent (among them, the mass percentage of manganese and alumina is 2%), used for NF 3 Anhydrous decomposition reaction, NF 3 See the appendix for the decomposition percentage data figure 1 .
[0035] NF 3 Decomposition reaction conditions: take the defluorination agent and put it into the reaction tube, put it into the reaction furnace, and feed the reaction gas 2%NF 3 / 98%He (NF 3 The volume concentration is 2%), and the gas space velocity is 1.5 liters / hour / gram (defluorination agent). Electric heating to 400 ℃, constant temperature reaction. Testing NF with Gas Chroma...
Embodiment 2
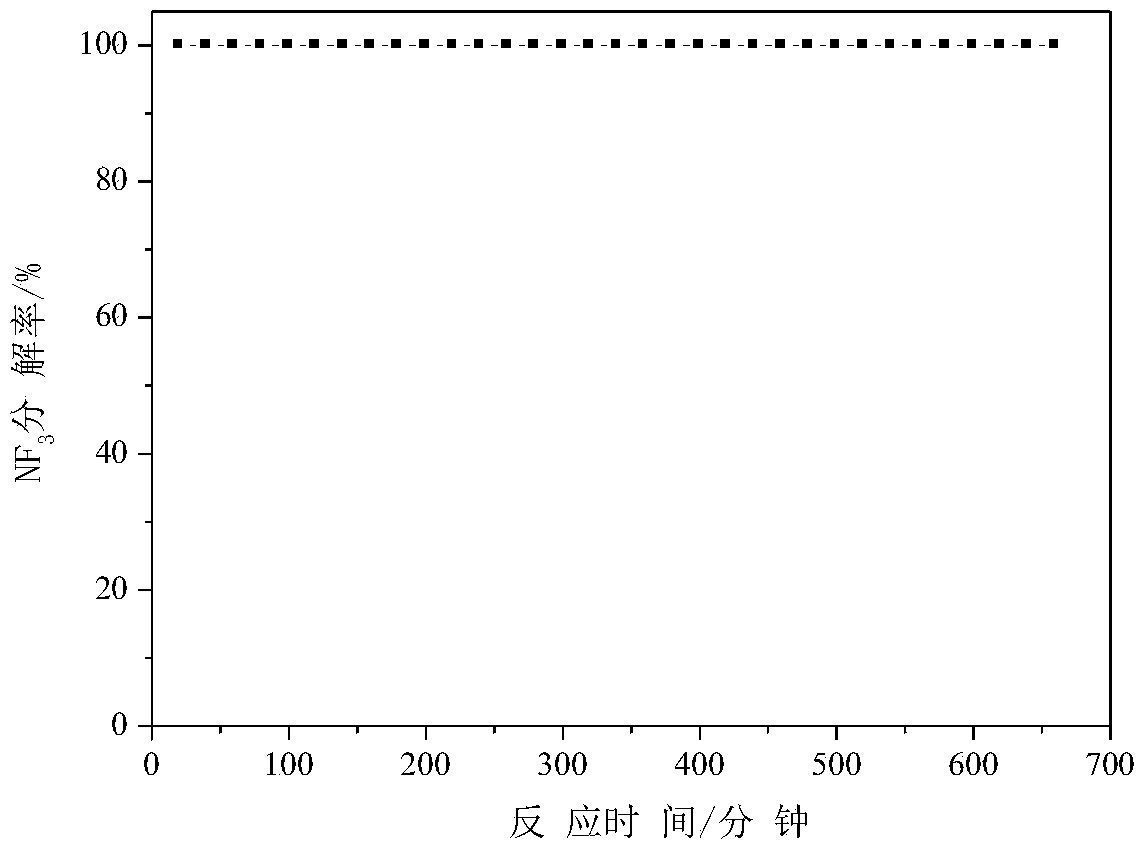
[0037] Weigh 1.301 g of manganese nitrate solution with a mass concentration of 50%, add water to dilute to 8.5 ml, impregnate 5 g of alumina particles for 24 hours, dry at 100°C for 6 hours, and roast at 600°C for 3 hours to obtain manganese oxide-alumina defluorination agent (among them, the mass percentage of manganese and alumina is 4%) for NF 3 Anhydrous decomposition reaction, NF 3 See the appendix for the decomposition percentage data figure 2 .
Embodiment 3
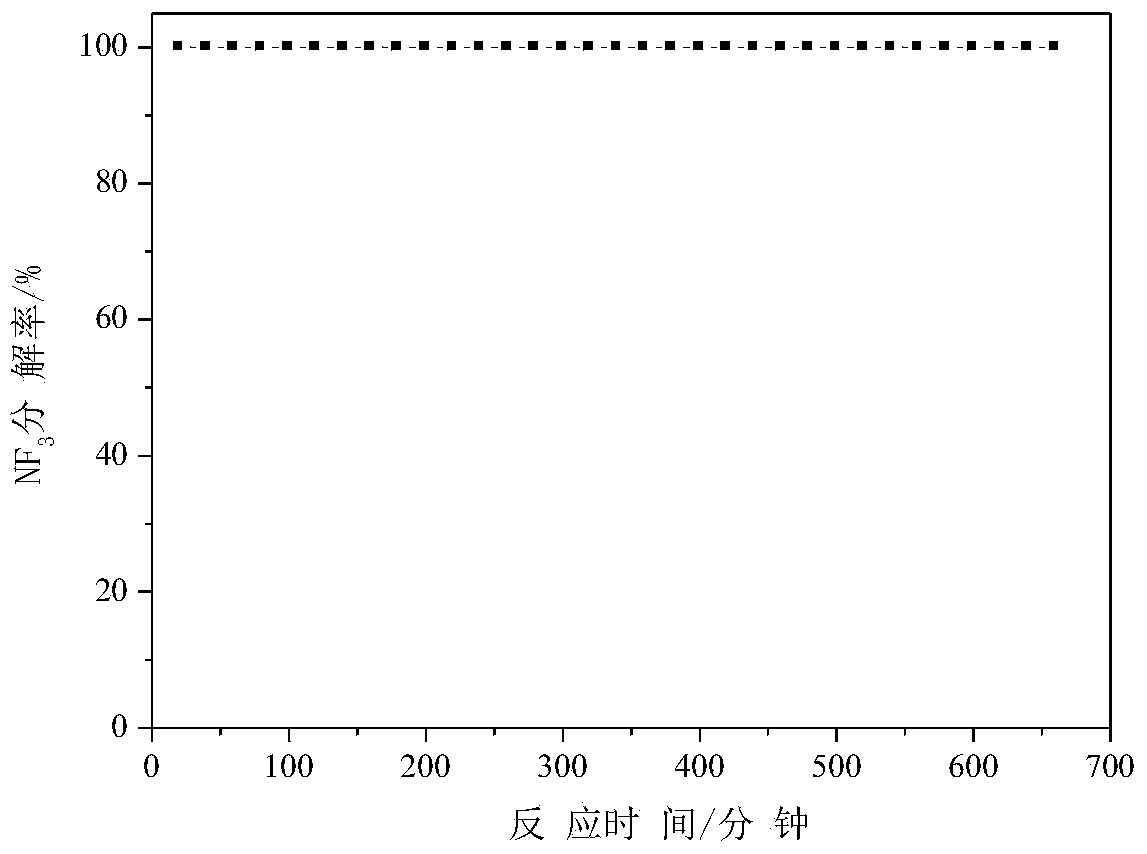
[0039] Weigh 1.626 g of manganese nitrate solution with a mass concentration of 50%, add water to dilute to 8.5 ml, impregnate 5 g of alumina particles for 24 hours, dry at 100°C for 6 hours, and roast at 500°C for 3 hours to obtain manganese oxide-alumina defluorination agent (among them, the mass percentage of manganese and alumina is 5%), used for NF 3 Anhydrous decomposition reaction, NF 3 See the appendix for the decomposition percentage data image 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com