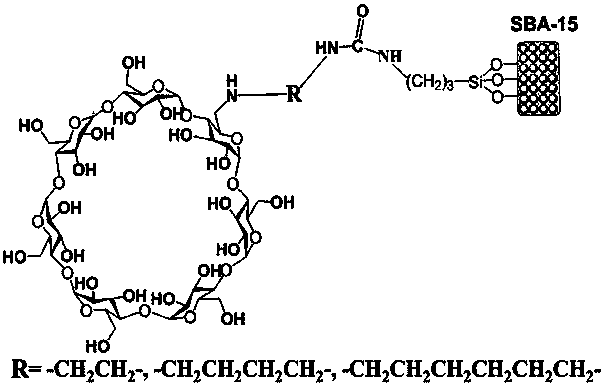
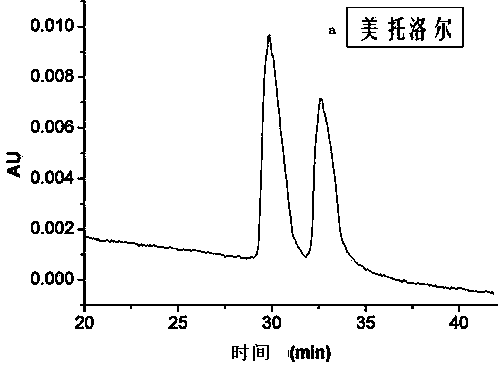
Preparation method and application of omega-diamine derivatization beta-cyclodextrin bonded SBA-15 chiral stationary phase
A technology of chiral stationary phase and cyclodextrin, which is applied in the chemical field, can solve the problems affecting chiral separation effect, batch-to-batch chromatographic separation performance of difficult chiral stationary phase, and cyclodextrin port congestion, etc., so as to improve chiral separation High energy, abundant chiral recognition sites, cheap price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Take SBA-15 (400 m 2 / g) Activated silica gel 2.5 g as the base.
[0034] (1) Under nitrogen atmosphere, according to the ratio of 6-p-toluenesulfonylated β-cyclodextrin (mmol):ethylenediamine (ml) of 1.0:15, 6-p-toluenesulfonylated β-cyclodextrin Add alcohol and ethylenediamine into a round bottom flask, stir for 0.5 h to fully dissolve the raw materials to form a homogeneous solution. Configure a condenser tube and a calcium chloride drying tube, and react with magnetic stirring for 4 h in an oil bath at 80 °C under nitrogen protection. Then evaporate the solvent under reduced pressure, dissolve the solid in a small amount of hot water, add acetone-water solution (10:1, v / v) under stirring, collect the white precipitate, and repeat the above operation 3 times, and dry in a vacuum oven at 40°C Finally, 6-ethylenediamino-β-cyclodextrin is obtained, and the reaction yield of this step is 60%;
[0035] (2) Under nitrogen atmosphere, according to 6-ethylenediamino β-cyc...
Embodiment 2
[0042] Take SBA-15 (500 m 2 / g) Activated silica gel 2.5 g as the base.
[0043] (1) Under nitrogen atmosphere, according to the ratio of 6-p-toluenesulfonylated β-cyclodextrin (mmol):ethylenediamine (ml) of 1.0:20, 6-p-toluenesulfonylated β-cyclodextrin Add alcohol and ethylenediamine into a round bottom flask, stir for 1 h to fully dissolve the raw materials to form a homogeneous solution. Configure a condenser tube and a calcium chloride drying tube, and react with magnetic stirring for 6 h in an oil bath at 85 °C under the protection of nitrogen. Then evaporate the solvent under reduced pressure, dissolve the solid in a small amount of hot water, add acetone-water solution (10:1, v / v) under stirring, collect the white precipitate, and repeat the above operation 3 times, and dry in a vacuum oven at 40°C Finally, 6-ethylenediamino-β-cyclodextrin is obtained, and the reaction yield of this step is 65%;
[0044] (2) Under a nitrogen atmosphere, according to 6-ethylenediamin...
Embodiment 3
[0051] Take SBA-15 (420 m 2 / g) Activated silica gel 2.5 g as the base.
[0052] (1) Under nitrogen atmosphere, according to the ratio of 6-p-toluenesulfonylated β-cyclodextrin (mmol): butanediamine (ml) is 1.0:16, 6-p-toluenesulfonylated β-cyclodextrin Add alcohol and butylenediamine into a round bottom flask, stir for 0.8 h to fully dissolve the raw materials to form a homogeneous solution. Configure a condenser tube and a calcium chloride drying tube, and react with magnetic stirring for 5 h in an oil bath at 85 °C under the protection of nitrogen. Then evaporate the solvent under reduced pressure, dissolve the solid in a small amount of hot water, add acetone-water solution (10:1, v / v) under stirring, collect the white precipitate, and repeat the above operation 3 times, and dry in a vacuum oven at 40°C Finally, 6-butanediamino-β-cyclodextrin was obtained, and the reaction yield of this step was 58%;
[0053] (2) Under nitrogen atmosphere, according to 6-butanediamino β...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com