An Alkaline Pectinase Mutant with Improved Secretion Performance
A technology of secretion performance and pectinase, which is applied in the field of bioengineering, can solve the problems of high-potency commercial alkaline pectinase, which cannot be ignored in research, and achieve enhanced heat resistance, increased expression of extracellular secretion, and enzymatic properties Improved effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The construction of embodiment 1 mutant expression plasmid and the acquisition of recombinant Bacillus subtilis
[0028] 1. Construct a mutant expression vector using the pET-20b(+)-pgl plasmid as a template
[0029] The nucleotide sequence of the gene encoding the wild-type alkaline pectinase and the signal peptide consisting of 21 amino acids is shown in SEQ ID NO.1, and the amino acid sequence of the wild-type mature alkaline pectinase is shown in SEQ ID NO.2. By analyzing the three-dimensional structure of alkaline pectinase, it is speculated that the isoleucine I at position 58 has a greater impact on the secretion and expression of alkaline pectinase, and a mutation experiment was designed to mutate the isoleucine I at position 58 into valine Acid V.
[0030] Using the pET-20b(+)-pgl plasmid as a template, the plasmid containing the mutant gene was amplified in vitro by PCR (polymerase chain reaction), and the isoleucine at position 58 was mutated into valine.
...
Embodiment 2
[0046] Expression of embodiment 2 mutant PGL
[0047] Seed medium composition (g / L): yeast powder 5, tryptone 10, NaCl 10, glucose 20, pH 7.0.
[0048] Composition of fermentation medium: yeast powder 24g / L, tryptone 12g / L, glycerol 5g / L, K 2 HPO 4 72mmolL -1 , KH 2 PO 4 17mmolL -1 .
[0049] Inoculate the recombinant bacteria E.coliBL21(DE3) (pET-20b(+)-pglI58V) containing the mutant expression vector pET-20b(+)-pglI58V into 100μgmL -1 In the seed medium of ampicillin, the filling volume is 20mL / 250mL. Culture temperature 37℃, 200rpmmin -1 Incubate on a shaker for 10 h.
[0050] The seed solution cultivated for 10h was inoculated with 100μgmL with a 3% (V / V) inoculum -1 In the fermentation medium of ampicillin, the filling volume is 50mL / 500mL, at 37°C, 200rmin -1 to cultivate. Bacteria grow to a certain stage (OD 600 =0.6), adding a final concentration of 0.4mMIPTG for induction, while adjusting the temperature to 30°C, and inducing fermentation for 48h.
Embodiment 3
[0051] Example 3 PGL extracellular enzyme activity before and after mutation
[0052] According to the method described in Example 2, E.coliBL21(DE3) containing the unmutated expression vector pET-20b(+)-pgl and the mutant strain E.coliBL21(DE3)(pET-20b(+)-pglI58V ) for fermentation.
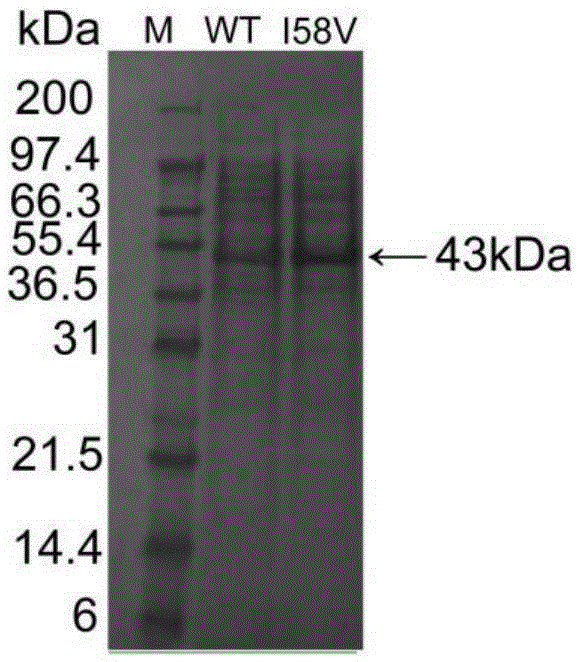
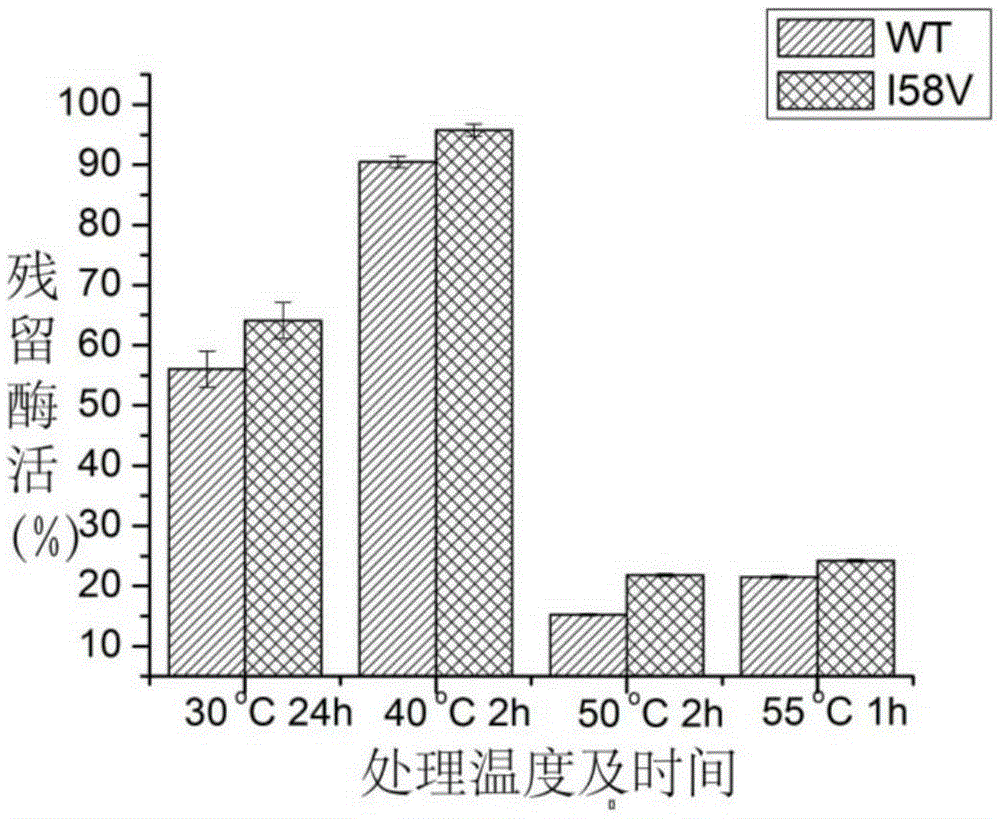
[0053] figure 1 SDS-PAGE protein electrophoresis showed that the extracellular secretion expression of alkaline pectinase I58V was significantly increased after mutation, the extracellular enzyme activity of alkaline pectinase was 129.65U / mL before mutation, and the extracellular enzyme activity of alkaline pectinase increased after mutation It was 337.58U / mL, which was 2.60 times that before the mutation. Combined with Table 1, the specific enzyme activity of alkaline pectinase after mutation increased from 179.14U / mg to 295.63U / mg, which was 1.65 times that before mutation. It shows that after the mutation, the secretion ability of alkaline pectinase I58V is enhanced, and the enzyme activit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com