Method for realizing separation of potassium-rich solution through hydrochloric acid coproduced by utilizing membrane electrolysis technology to mineralize CO2
A technology of membrane electrolysis and catholyte, applied in the direction of electrolysis process, electrolysis components, etc., can solve the problems of small processing capacity, poor separation effect, high cost, etc., and achieve the effect of low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
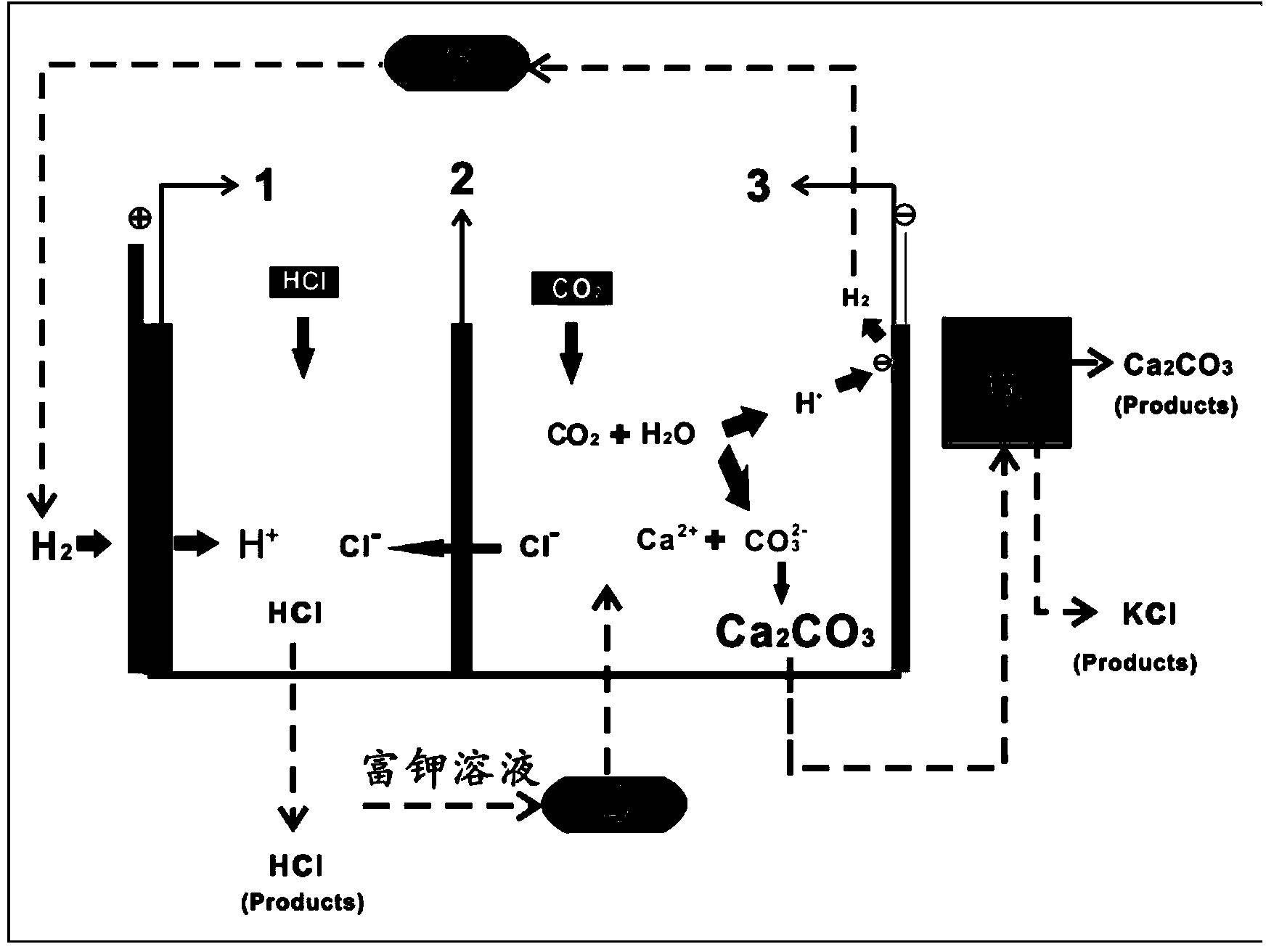
[0022] The mineralization process of the present embodiment is as attached figure 1 shown. The electrolytic cell is divided into two areas, the anode area and the cathode area, by the anion exchange membrane 2 which only allows the anion to pass through but can prevent the cation from passing through. Add 0.3mol / L HCl solution to the anode electrolyzer as the anolyte, and add calcium chloride concentration of 1mol / L and potassium chloride concentration of 1.2mol / L to the cathode electrolyzer through the potassium-rich solution storage tank 4 The potassium-rich solution acts as the catholyte and is the starting material for the electrolysis reaction. A gas diffusion electrode is used as the anode electrode 1 , and a metal nickel electrode is used as the cathode electrode 3 . will CO 2 The gas is passed into the cathode area by bubbling from the bottom of the electrolytic cell, and the hydrogen gas generated by the cathode electrode is collected and entered into the buffer ta...
Embodiment 2
[0024] The mineralization process of the present embodiment is as attached figure 1 shown. The electrolytic cell is divided into two areas, positive and negative, by an anion exchange membrane 2 that only allows the penetration of anions but prevents the penetration of cations. Add 0.5 mol / L HCl solution to the anode electrolyzer as the anolyte, add a potassium-rich solution with a calcium chloride concentration of 2 mol / L and a potassium chloride concentration of 1 mol / L as the catholyte and the raw material for the electrolysis reaction. A hydrogen diffusion electrode is used as the anode electrode 1 , and a metal nickel electrode is used as the cathode electrode 3 . will CO 2 The gas is bubbled at the bottom of the electrolytic cell into the cathode area, and the hydrogen gas generated by the cathode electrode is collected and entered into the buffer tank 5. The hydrogen gas from the buffer tank is passed into the gas diffusion electrode. Under these conditions, the elec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com