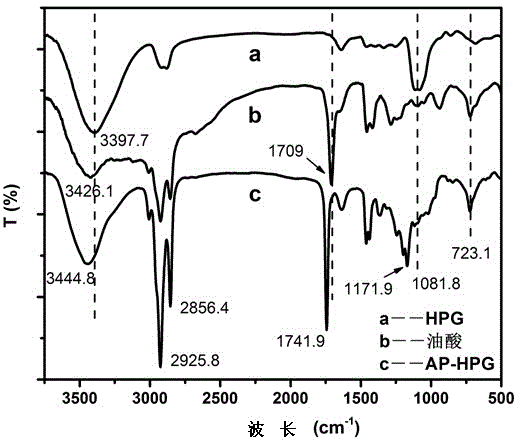
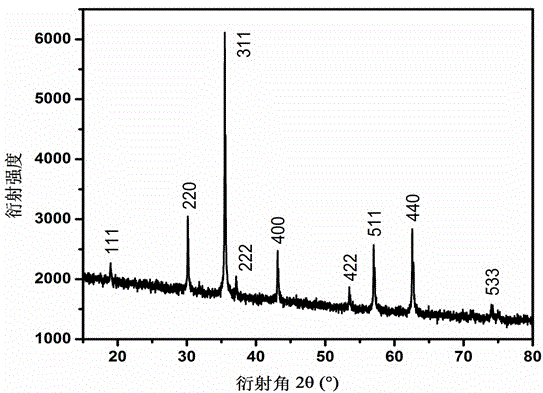
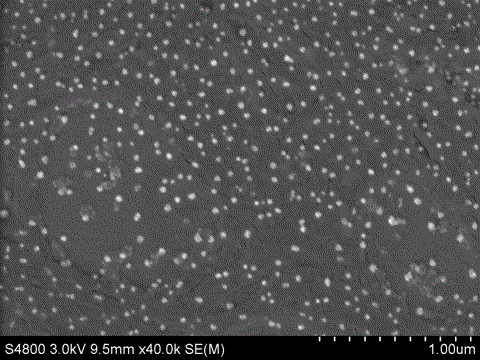
A method for preparing iron ferric oxide nanoparticles
A technology of ferroferric oxide and nanoparticles, which is applied in the field of nanomaterials to achieve the effects of high crystallinity, good dispersion and stability, and high surface activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Dissolve 6.0 mL of oleoyl chloride in 60 mL of toluene, and dissolve 2 g of hyperbranched polyglycidyl ether in 80 mL of pyridine. The obtained oleoyl chloride toluene solution is dropped dropwise into the pyridine solution of hyperbranched polyglycidyl ether. After stirring at 30°C for 48 hours, The excess solvent was removed by rotary evaporation at 80°C, and the excess oleoyl chloride was removed by washing with chloroform to obtain amphiphilic hyperbranched polyglycidyl ether.
[0028] Take (NH 4 ) 2 Fe(SO 4 ) 2 .6H 2 0.784g was dissolved in 20mL deionized water, 0.5g amphiphilic hyperbranched polyglycidyl ether was dissolved in 10mL chloroform, and under rapid stirring, the amphiphilic hyperbranched polyglycidyl ether solution was slowly added dropwise to (NH 4 ) 2 Fe(SO 4 ) 2 .6H 2 O aqueous solution, mixed evenly to get Fe 2+ Precursor solution; 1g NaOH was dissolved in 10mL ethanol, and the resulting NaOH solution was added dropwise to the above Fe 2+ ...
Embodiment 2
[0035] Take 1.0 mL of oleoyl chloride and dissolve in 80 mL of toluene, and dissolve 1 g of amino-terminated hyperbranched polymer in 100 mL of pyridine. The obtained oleoyl chloride toluene solution is dropped dropwise into the pyridine solution of amino-terminated hyperbranched polymer, and after stirring at 25 ° C for 40 h, The excess solvent was removed by rotary evaporation at 80°C, and the excess oleoyl chloride was removed by washing with acetone to obtain an amphiphilic amino-terminated hyperbranched polymer.
[0036] FeSO 4 ·7H 2 O1.112g was dissolved in 40mL deionized water, 1g amphiphilic amino-terminated hyperbranched polymer was dissolved in 20mL ethanol, and under rapid stirring, the amphiphilic amino-terminated hyperbranched polymer solution was slowly added dropwise to FeSO 4 ·7H 2 O aqueous solution, mixed evenly to get Fe 2+ Precursor solution; 2g KOH was dissolved in 20mL n-butanol, and the resulting KOH solution was added dropwise to the above Fe 2+ In ...
Embodiment 3
[0038] Dissolve 5.0 mL of oleoyl chloride in 40 mL of diethyl ether, and dissolve 2 g of hyperbranched polyglycidyl ether in 50 mL of methanol. The obtained oleoyl chloride diethyl ether solution is dropped dropwise into the methanol solution of hyperbranched polyglycidyl ether. After stirring at 50°C for 56 hours, Rotary evaporation at 50°C removes excess solvent, washes with ethyl acetate to remove excess oleoyl chloride, and vacuum-dries at 60°C for 12 hours to obtain amphiphilic hyperbranched polyglycidyl ether.
[0039] FeCl 2 4H 2 O0.3976g was dissolved in 20mL deionized water, 0.5g amphiphilic hyperbranched polyglycidyl ether was dissolved in 10mL ethylene glycol, and under rapid stirring, the amphiphilic hyperbranched polyglycidyl ether solution was slowly added dropwise to FeCl 2 4H 2 O aqueous solution, mixed evenly to get Fe 2+ Precursor solution; 15mL concentration of 28% ammonia solution was added dropwise to the above Fe 2+ In the precursor solution, the obta...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com