Method for synthesizing diisoborneol oxalate
A technology for synthesizing diisobornyl oxalate and diisobornyl oxalate, which is applied to the preparation of carboxylic acid esters, chemical instruments and methods, and the preparation of organic compounds, and can solve the lack of synthetic methods for diisobornyl oxalate products , Difficulty in synthesizing borneol, etc., to achieve the effect of easy purification, easy acquisition, and low operating cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
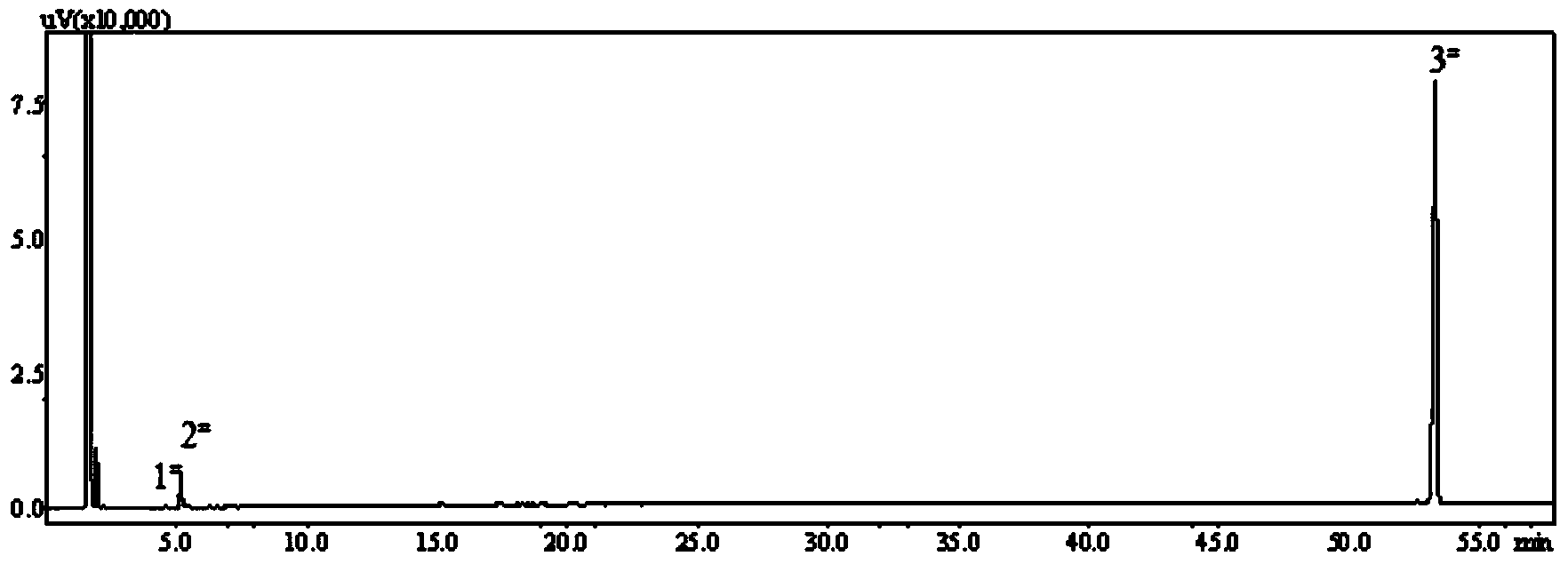
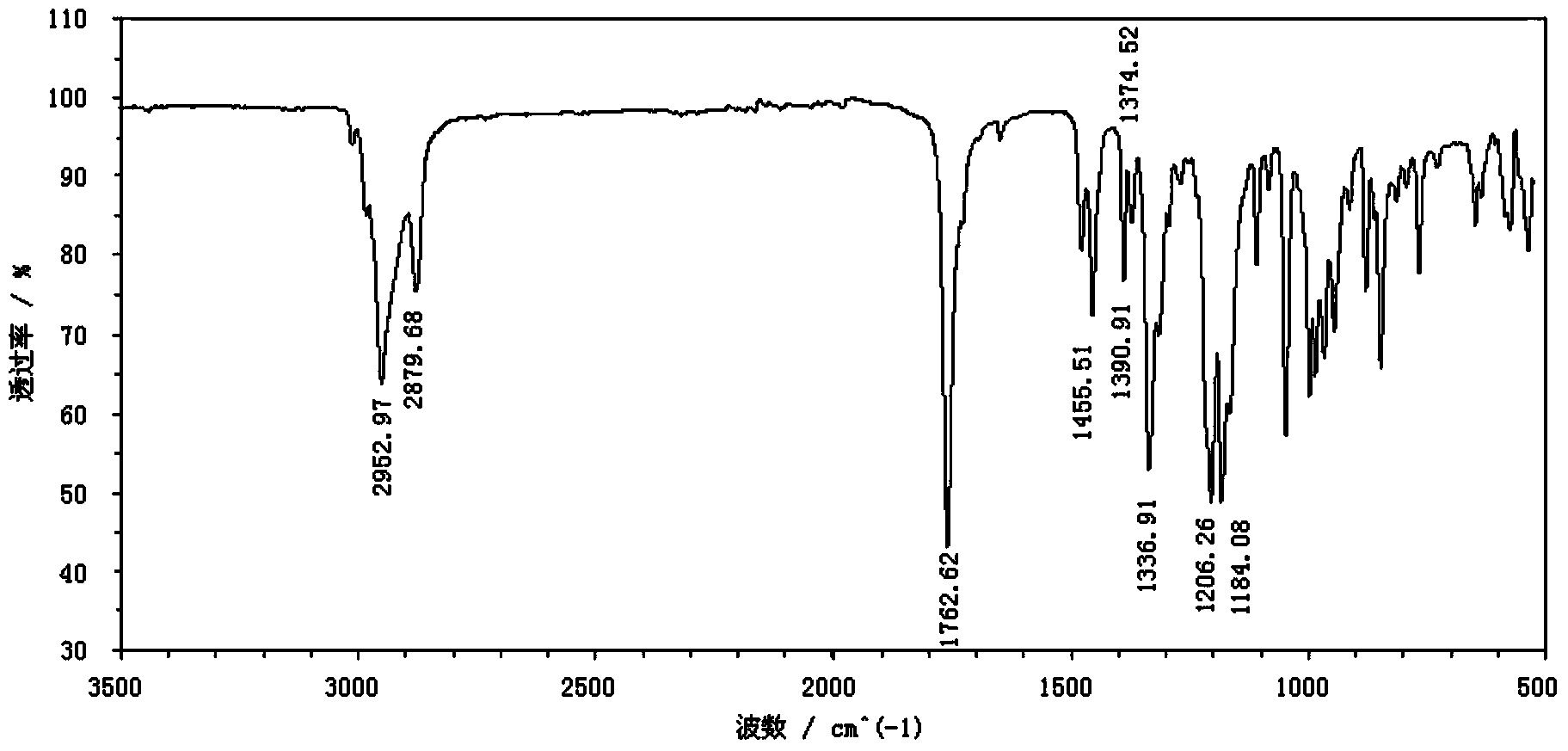
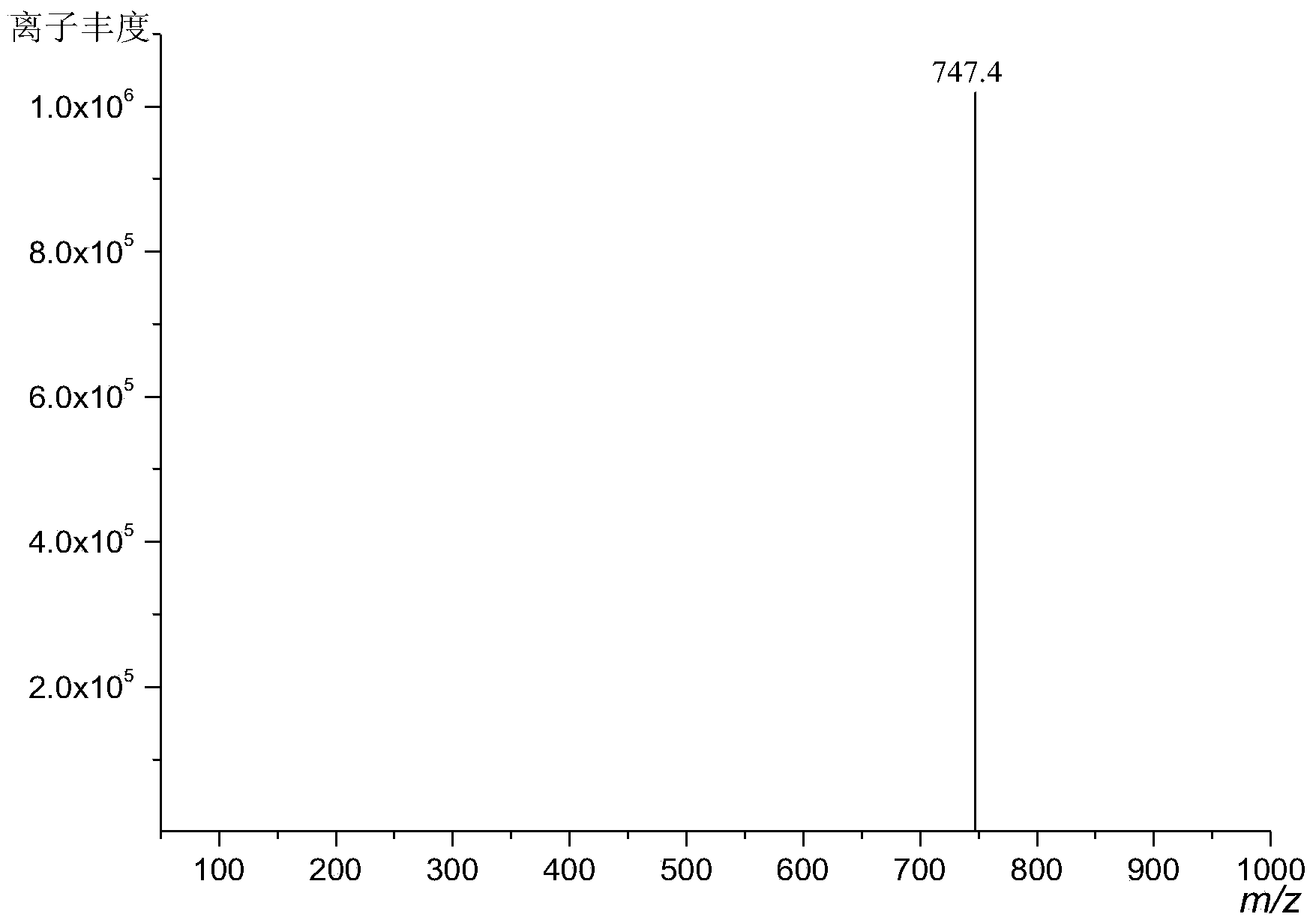
Image
Examples
Embodiment 1
[0045] Embodiment 1 (with industrial camphene as raw material)
[0046] With 10.3g (0.076mol) amphene (produced by Suzhou Youhe Technology Co., Ltd., its composition and content are: 77.0% amphene, 20.1% tricyclene, specific optical rotation ) was dissolved in 107.5g of light petroleum ether, reacted with 8.1g (0.090mol, the mass ratio of amphene to amphene: 1.18) of anhydrous oxalic acid under the action of 1.1g of boron anhydride catalyst for 72h, and filtered to remove The catalyst and unreacted oxalic acid were washed 5-6 times with water below 30°C, and the upper liquid was removed by rotary evaporation to remove most of the solvent, and then steam distillation was used to remove unreacted raw material camphene, tricycloene and a small amount of by-products. The product isoborneol is obtained thick product, then obtains diisoborneol oxalate 2.6g (0.0072mol, yield 19.0%) through repeated recrystallization with acetone, ethanol, ethyl acetate, normal hexane or hexanaphthen...
Embodiment 2
[0048] 29.0g (0.21mol) of camphene is dissolved in 190.0g of light petroleum ether, and 16.1g (0.18mol, the substance mass ratio with camphene is 0.84) of anhydrous oxalic acid under the action of 2.7g boron anhydride catalyst is at 55 ℃ After reacting for 85 hours, other conditions and operations were the same as in Example 1 to obtain 11.3 g (0.031 mol, yield 29.2%) of diisobornyl oxalate.
Embodiment 3
[0050] 11.0g (0.081mol) amphene was dissolved in 151.1g light petroleum ether, and 11.0g (0.12mol, the mass ratio with amphene was 1.51) anhydrous oxalic acid under the action of 0.7g boron anhydride catalyst at 55 ℃ After reacting for 191 h, other conditions and operations were the same as in Example 1, and 2.1 g (0.0058 mol, yield 14.2%) of relatively pure diisobornyl oxalate was obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com