Method for measuring residual acetic acid in flubendazole crude drug by high performance liquid chromatography method
A high-performance liquid chromatography and flubenimidazole technology, which is applied in the field of chemical detection, can solve problems such as large dosage and affect product quality, and achieve the effects of simple operation and accurate and reliable measurement results.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Method for determination of residual acetic acid in flubendazole bulk drug by high performance liquid chromatography:
[0031] Instruments and reagents: LC-2010A high performance liquid chromatography (quaternary pump, degassing unit, UV detector, column oven, automatic sampler, system monitor, LCsolution) (Shimadzu Corporation of Japan); (Manufacturer: Jiangsu Baozhong Baoda Pharmaceutical Co., Ltd., methanol (Merck chromatographically pure), other reagents are analytically pure, and water is self-made fresh redistilled water.
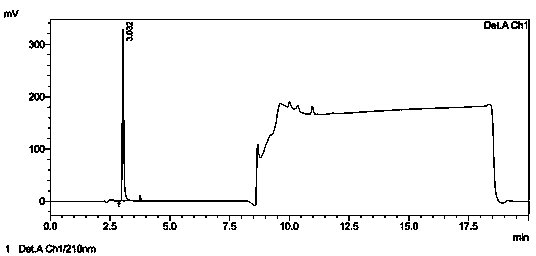
[0032] Chromatographic condition selection: Chromatographic column: ZORBAX SB—C 18 (4.6×250mm, 5μm); mobile phase A: buffer-methanol=950:50 (the buffer is 0.7mL phosphoric acid in 1000mL water, adjust the pH to 3.0 with 1mol?L sodium hydroxide), mobile phase B: methanol, Gradient elution; detection wavelength: 210nm; calculation method: external standard method; flow rate: 1.2 mL?min -1 ; Sensitivity: 1.0 AUFS; Injection volume: 20 μL; Column...
Embodiment 2
[0041] The stability test of the inventive method:
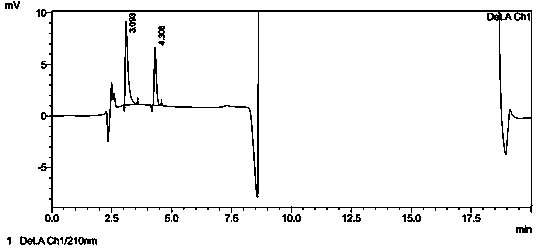
[0042] Take the control solution under Example 1, place it at room temperature for 0, 2, 4, 6, 8, and 10 hours respectively, and then inject samples according to the method in the example. The injection volume is 20 μL. After calculation, the peak area of acetic acid is The RSD is 0.39%, which shows that the method has good stability.
Embodiment 3
[0044] Quantitation limit test of the inventive method:
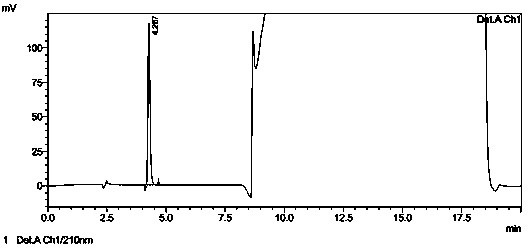
[0045] Weigh 100mg of glacial acetic acid, add water to dissolve and dilute to contain glacial acetic acid 5μg?mL -1 The solution of the sample is injected according to the method of the embodiment, and the sample injection is 2 times, and each injection volume is 20 μ L, and the chromatogram is recorded, referring to Figure 5 , the peak height of acetic acid in the chromatogram is about 8.7 times of the baseline noise (ie S / N=8.7), then the limit of quantitation of this method is 5 μg?mL -1 , converted to 515ppm according to the concentration of flubendazole sample preparation.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com