Preparation method of ZnCo2O4 nano-plate serving as lithium ion battery negative electrode material
A lithium-ion battery and negative electrode material technology, applied in battery electrodes, nanotechnology for materials and surface science, nanotechnology, etc., can solve the problems of negative electrode material capacity decay, short cycle life, poor rate performance, etc. The effect of mild conditions, low cost and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] (1) Dissolve zinc nitrate and cobalt nitrate with a molar ratio of 1:2 in a high-purity water solvent, stir magnetically for 30 minutes, and after filling and dissolving, add a certain amount of Sodium hydroxide reagent, after mixing, continue to stir for 10 to 30 minutes.
[0024] (2) Transfer the above mixed solution to a microwave reaction vessel, set the power to 500W, and condense and reflux for 30 minutes under microwave irradiation. After the reaction is completed, naturally cool to room temperature, centrifuge, wash, separate and dry to obtain the zinc cobaltate precursor bulk nanomaterials.
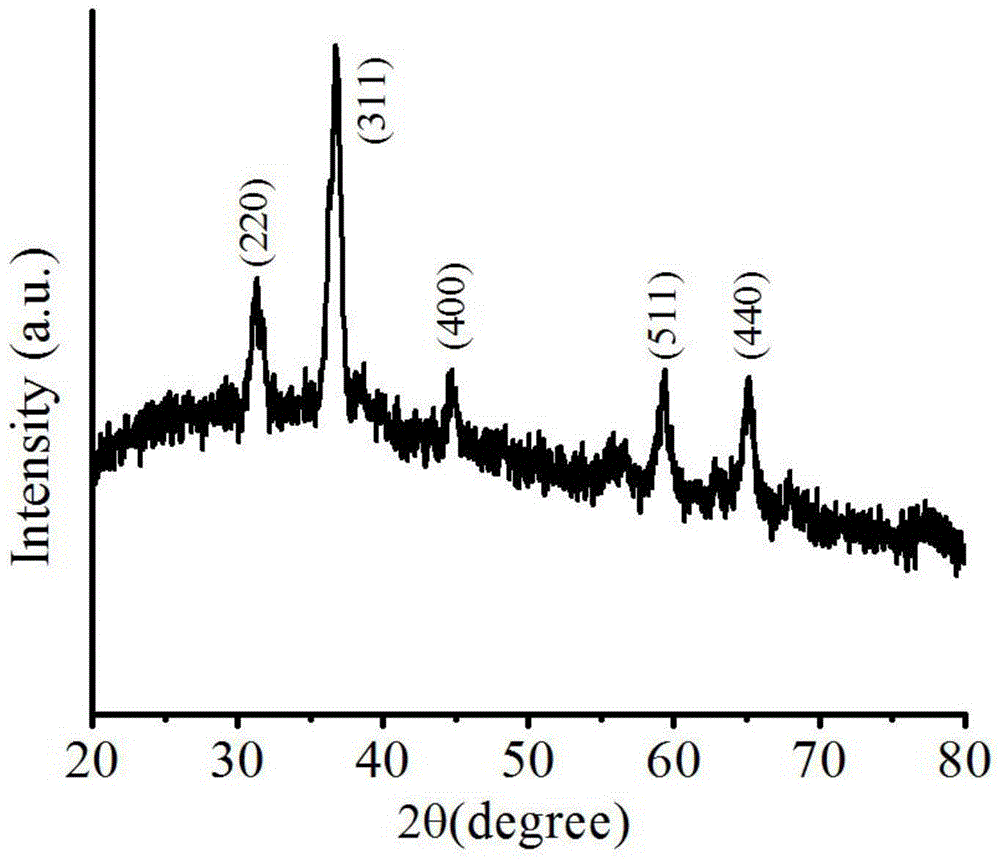
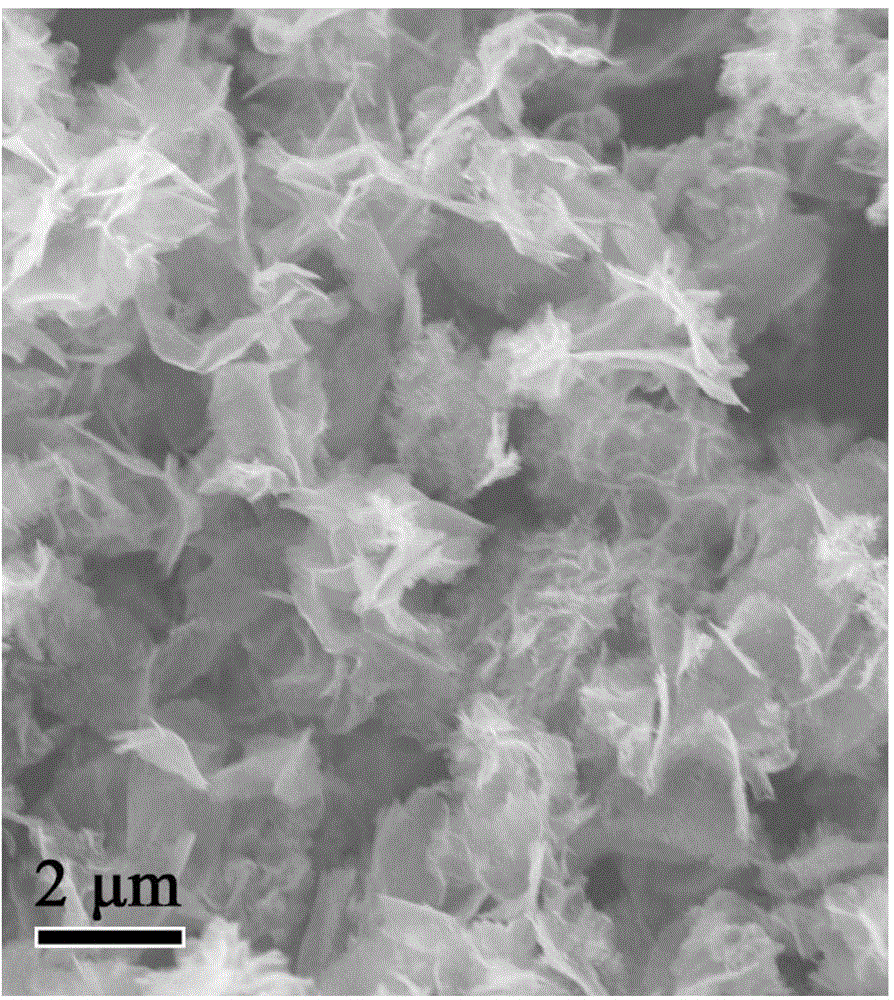
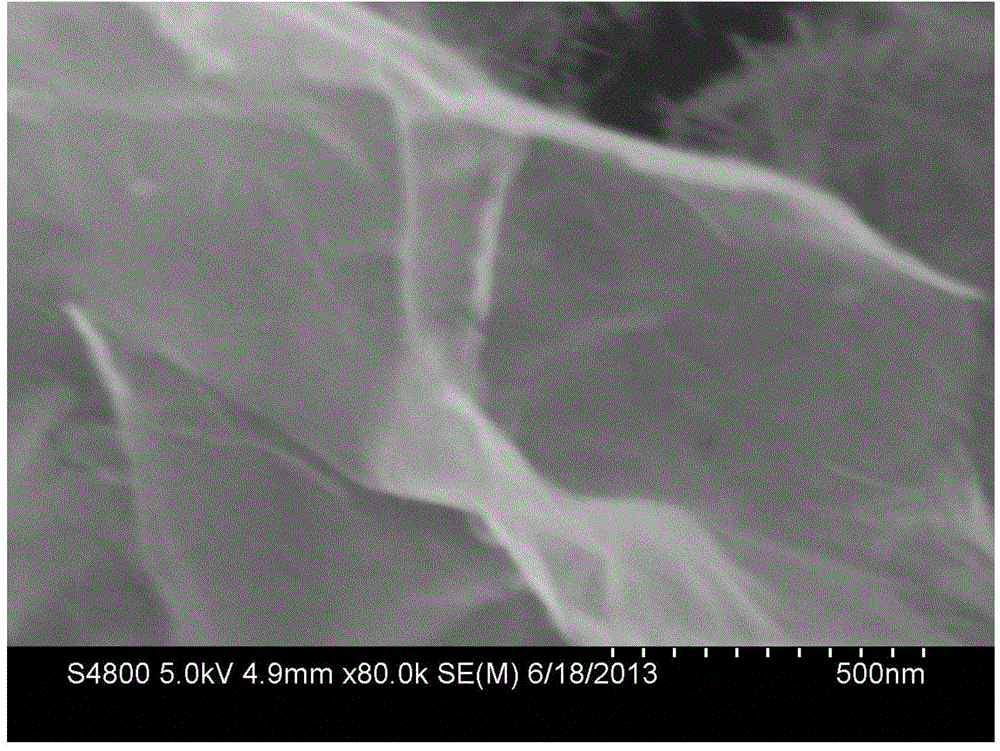
[0025] (3) Put the zinc cobaltate precursor nanomaterial obtained in the previous step into a high-temperature furnace for 3 hours at 350°C for heat treatment, and then naturally cool to room temperature to obtain a two-dimensional ultra-thin ZnCo 2 o 4 Nanosheets.
[0026] (4) Use the material prepared in the previous step as the negative electrode of lithium ion batte...
Embodiment example 2
[0029] (1) Dissolve zinc sulfate and cobalt acetate with a molar ratio of 1:2 in a mixed solvent of high-purity water and ethylene glycol, wherein the volume ratio of water and ethylene glycol is 1:7, stir magnetically for 30 minutes, and wait for the solution to dissolve. , and then add a certain amount of potassium hydroxide reagent according to the molar ratio of metal ion to alkaline reagent is 1:8, mix and continue to stir for 10 to 30 minutes.
[0030] (2) Transfer the above mixed solution to a microwave reaction vessel, set the power to 500W, and condense and reflux for 15 minutes under microwave irradiation. After the reaction is completed, naturally cool to room temperature, centrifuge, wash, separate and dry to obtain the zinc cobaltate precursor bulk nanomaterials.
[0031] (3) Put the zinc cobaltate precursor nanomaterial obtained in the previous step into a high-temperature furnace at 350 °C for 2 hours, and then naturally cool to room temperature to obtain a two-...
Embodiment example 3
[0034] (1) Dissolve zinc nitrate and cobalt chloride with a molar ratio of 1:2 in ethylene glycol solvent, and stir magnetically for 30 minutes. After filling and dissolving, then add it according to the molar ratio of metal ion and alkaline reagent as 1:4 A certain amount of urea reagent, after mixing, continue to stir for 10 to 30 minutes.
[0035] (2) Transfer the above mixed solution to a microwave reaction vessel, set the power to 700W, and condense and reflux for 10 minutes under microwave irradiation. After the reaction is completed, naturally cool to room temperature, centrifuge, wash, separate and dry to obtain the zinc cobaltate precursor bulk nanomaterials.
[0036] (3) Put the zinc cobaltate precursor nanomaterial obtained in the previous step into a high-temperature furnace at 400 °C for 2 hours, and then naturally cool to room temperature to obtain a two-dimensional ultra-thin ZnCo 2 o 4 Nanosheets.
[0037] (4) After the battery is assembled according to the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com