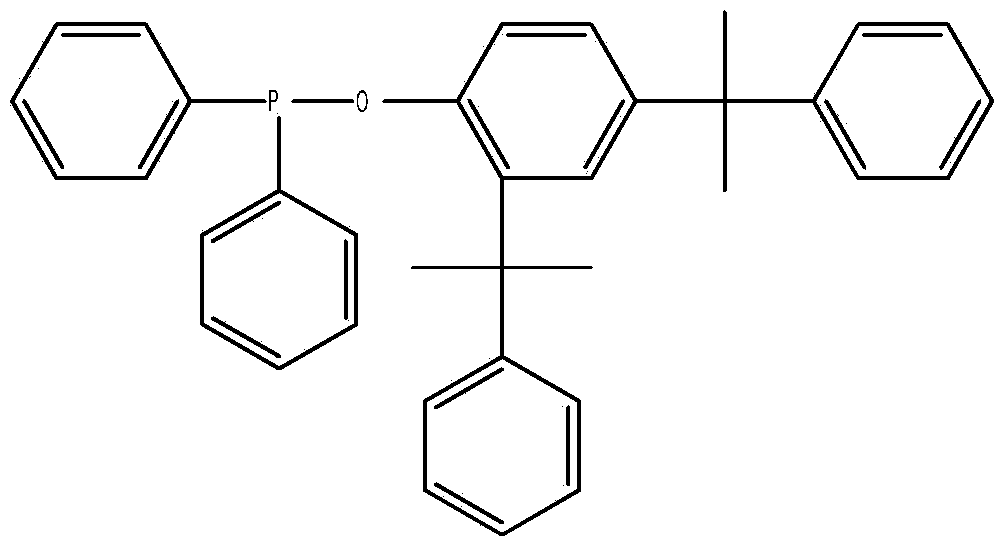
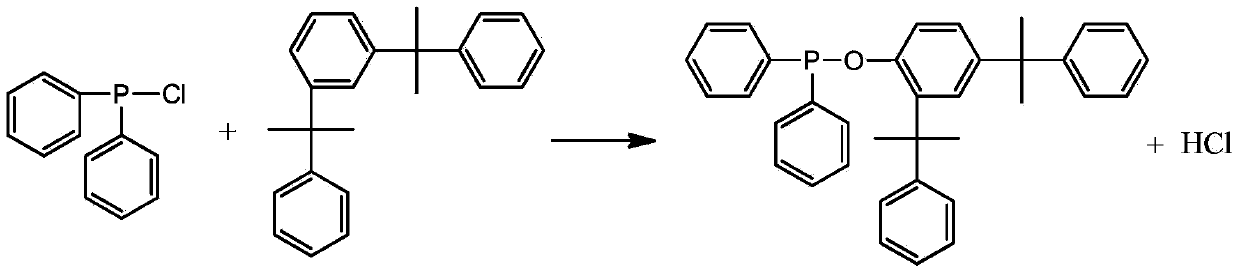
Diphenylphosphine cumyl phenolic ester and synthetic method thereof
A diphenylphosphine and synthesis method technology, applied in the field of phosphite antioxidants, can solve the problems of increased product cost, complicated operation steps, complicated operation, etc., achieve high thermal decomposition temperature, simplify operation steps, and process is environmentally friendly Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Add 31.4 grams of cumol and 100 ml of toluene into a 500 ml three-necked flask, and pass in nitrogen gas. After stirring evenly, slowly add a mixture of 22 grams of diphenylphosphine chloride and 20 mL of toluene under the condition of controlling the temperature at 80°C. , kept at 80°C for 4 hours, lowered the temperature, stopped passing nitrogen, evaporated toluene under reduced pressure to obtain a yellow viscous substance, added 40mL of ethanol / acetone (V / V=1:1) and 0.5g of activated carbon, refluxed for 30 minutes, and After hot filtration and cooling, the precipitated solid was filtered and dried to obtain the product with a yield of 85.3% based on diphenylphosphine chloride. The product is analyzed by liquid chromatography with a purity of 99.3%, a 1% thermal weight loss temperature of 351°C, and a phosphorus content of 6.06%.
Embodiment 2
[0030] Add 31.4 grams of cumol and 100 ml of toluene into a 500 ml three-necked flask, pass in nitrogen gas, stir evenly, and slowly add a mixture of 22 grams of diphenylphosphine chloride and 20 ml of toluene under the condition of controlling the temperature at 80°C. , heat up to 100°C and keep warm for 10 hours, cool down, stop nitrogen flow, evaporate toluene under reduced pressure to obtain a yellow sticky substance, add 40mL of ethanol / acetone (V / V=1:1) and 0.5g of activated carbon, and keep at reflux for 30 minutes , filtered while hot, and after cooling, the precipitated solid was filtered and dried to obtain the product, and the yield was 92.6% based on diphenylphosphine chloride. The product is analyzed by liquid chromatography with a purity of 99.4%, a 1% thermal weight loss temperature of 352°C, and a phosphorus content of 6.02%.
Embodiment 3
[0032] Add 31.4 grams of cumol and 100 ml of benzene into a 500 ml three-necked flask, and pass in nitrogen gas. After stirring evenly, slowly add a mixture of 22 grams of diphenylphosphine chloride and 20 ml of benzene under the condition of controlling the temperature at 100°C. , kept at 100°C for 4 hours, lowered the temperature, stopped passing nitrogen, evaporated toluene under reduced pressure to obtain a yellow viscous substance, added 40mL of ethanol / acetone (V / V=1:1.5) and 0.5g of activated carbon, refluxed for 30 minutes, while After hot filtration and cooling, the precipitated solid was filtered and dried to obtain the product with a yield of 88.7% based on diphenylphosphine chloride. The product is analyzed by liquid chromatography with a purity of 99.1%, a 1% thermal weight loss temperature of 349°C, and a phosphorus content of 6.10%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com