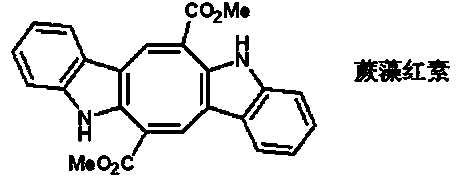
Method for synthesizing caulerpin
A technology for synthesizing phycoerythrin and phycoerythrin, which is applied in organic chemistry and other fields, can solve the problems of low yield and achieve the effects of easy-to-obtain raw materials, mild reaction conditions, and easy synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
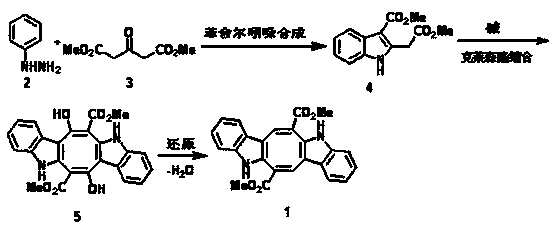
[0032] Step 1: Dimethyl 3-oxo-1,5-pentanedioate (17.4 g) was added dropwise to phenylhydrazine (10.8 g) under magnetic stirring at room temperature. Two drops of acetic acid were then added and stirring was continued for 3 hours. Concentrated sulfuric acid (20 ml) was added dropwise to the above reactant, stirred for 30 minutes, poured into crushed ice (200 g), and a pale yellow solid was precipitated, which was identified as 2-(3'-methoxycarbonyl)indole - methyl acetate (compound 4). 1 H NMR ( CDCl 3 , 400MHz ): δ 10.97 (bs, 1H), 8.08-8.04 (m, 1H), 7.49-7.46 ( m, 1H), 7.22-7.16 ( m, 2H ), 4.32 ( s, 2H ), 3.92 ( s, 3H ), 3.67 ( s, 3H ). 13 C NMR (CDCl 3 , 100MHz ): δ 171.39, 166.11, 138.56, 134.78, 126.36, 122.91, 121.92, 121.51, 111.17, 105.21, 52.46, 50.91, 32.10.
[0033] Step 2: Dissolve 2-(3'-methoxycarbonyl)indole-acetic acid methyl ester (2.5 g) in methanol (25 ml), add sodium methoxide (0.6 g), stir at room temperature for 3 days, acidify with concentrated hydroch...
Embodiment 2
[0036] Step 1: Dimethyl 3-oxo-1,5-pentanedioate (17.4 g) was added dropwise to phenylhydrazine (8.8 g) under magnetic stirring at room temperature. Then two drops of formic acid were added and stirring was continued for 1 hour. Concentrated sulfuric acid (20 ml) was added dropwise to the above reactant, stirred at 50°C for 30 minutes, poured into crushed ice (200 g), and a pale yellow solid was precipitated, which was identified as 2-(3'-methoxycarbonyl ) indole-methyl acetate.
[0037] Step 2: Dissolve 2-(3'-methoxycarbonyl)indole-acetic acid methyl ester (2.5 g) in methanol (25 ml), add sodium methoxide (0.3 g), stir at 65°C for 3 days, and use Acidify with concentrated hydrochloric acid to pH=5. Concentrate on a rotary evaporator, remove 3 / 4 volume of solvent, add water (5 ml) dropwise to the residue, and precipitate a light gray precipitate, which is identified as an intermediate 5 .
[0038] Step 3: Intermediates 5 (2 g) was dissolved in methanol (20 ml), and palladi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com