Process of producing sodium hydrogenfluoride by using fluosilicic acid
A technology using fluosilicic acid and sodium bifluoride, which is applied in the chemical industry, can solve the problems of high cost of raw materials and insufficient quality of sodium bifluoride products, and achieve the effects of reducing raw material costs, increasing particle size, and ensuring quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
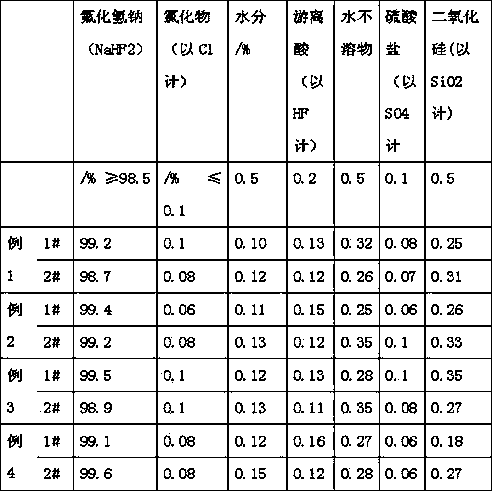
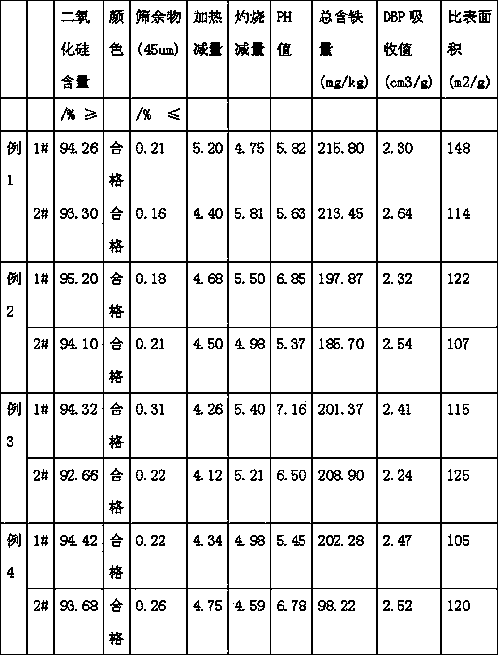
Examples
Embodiment 1
[0030] 1. After beating the soda ash, pour 20% of the amount of fluorosilicic acid required by 0.9 times the amount of soda ash into the reaction kettle at a constant speed, start stirring, and add silicon complexing agent (sodium nitrilotriacetate) of 0.2% of the amount of fluorosilicic acid ), turn on the steam, fully react, and the pH value at the end of the reaction is 8.5-9.
[0031] 2. After the reaction in the reactor is complete, the solid-solid separation is carried out by using the specific gravity difference reaction solution, and the solid-solid separation is sodium fluoride and silicon dioxide. Silica can be sold as white carbon black after pressure filtration and drying. After sodium fluoride centrifugation, it is dried and enters the next step.
[0032] 3. After drying, use sodium fluoride for beating, add 0.1% seed crystal (325 mesh sodium hydrogen fluoride), feed anhydrous hydrogen fluoride, and feed steam to control the temperature not lower than 60°C and co...
Embodiment 2
[0035] 1. After beating the soda ash, pour 20% of the amount of fluorosilicic acid required by 0.9 times the amount of soda ash into the reaction kettle at a constant speed, start stirring, and add silicon complexing agent (sodium nitrilotriacetate) of 0.2% of the amount of fluorosilicic acid ), turn on the steam, fully react, and the pH value at the end of the reaction is 8.5-9.
[0036] 2. After the reaction in the reactor is complete, the solid-solid separation is carried out by using the specific gravity difference reaction solution, and the solid-solid separation is sodium fluoride and silicon dioxide. Silica can be sold as white carbon black after pressure filtration and drying. After sodium fluoride centrifugation, it is dried and enters the next step.
[0037] 3. After drying, use sodium fluoride for beating, add 0.15% seed crystal (325 mesh sodium hydrogen fluoride), feed anhydrous hydrogen fluoride, and feed steam to control the temperature not lower than 60°C and c...
Embodiment 3
[0040] 1. After beating the soda ash, pour 30% of the amount of fluorosilicic acid required by 0.9 times the amount of soda ash into the reaction kettle at a constant speed, start stirring, and add silicon complexing agent (sodium nitrilotriacetate) of 0.2% of the amount of fluorosilicic acid ), turn on the steam, fully react, and the pH value at the end of the reaction is 8.5-9.
[0041] 2. After the reaction in the reactor is complete, the solid-solid separation is carried out by using the specific gravity difference reaction solution, and the solid-solid separation is sodium fluoride and silicon dioxide. Silica can be sold as white carbon black after pressure filtration and drying. After sodium fluoride centrifugation, it is dried and enters the next step.
[0042] 3. After drying, use sodium fluoride for beating, add 0.1% seed crystal (325 mesh sodium hydrogen fluoride), feed anhydrous hydrogen fluoride, and feed steam to control the temperature not lower than 60°C and co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com