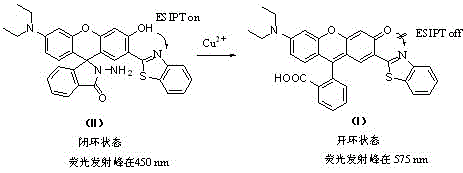
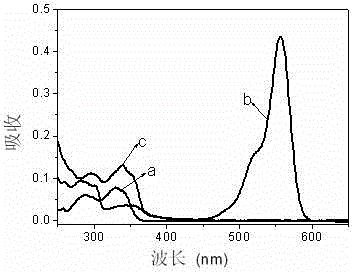
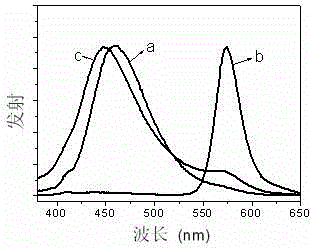
Xanthene dye, and preparation method and application thereof
A technology of condensation reaction and reaction temperature, which is applied in the field of xanthene dyes and its preparation and application, can solve the problems of ratio probe molecules, difficulties in large-scale promotion and use, cumbersome steps, etc., and achieve suppression of side reactions and good fluorescence The effect of labeling ability and simple operation of the experiment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 Preparation of compound (I)
[0037] (1) In a dry 50 mL round bottom flask, add 0.446 g (3.16 mmol) 2,4-dihydroxybenzaldehyde, 0.33 mL (3.12 mmol) o-aminothiophenol, 10 mL anhydrous DMF, and reflux for 2 h , after the reaction is complete, cool it to room temperature, then slowly drop it into 200 mL of cold water, precipitate out, filter, recrystallize with methanol, and dry to get 2-(2′,4′-dihydroxyphenyl)benzene And thiazole.
[0038] (2) In a dry 50 mL round bottom flask, add 0.991 g (6.00 mmol) of 3-diethylaminophenol, 1.066 g (7.20 mmol) of phthalic anhydride, and 15 ml of toluene. The reaction temperature was controlled at 80°C for 10 h, raised to 90°C for 5 h, then raised to 100°C for 2 h, and finally raised to 110°C for 1 h. After the reaction is complete, cool down, filter to obtain the crude product, then wash the crude product with n-butanol, recrystallize once, and dry to obtain 2-(4'-diethylamino-2'-hydroxybenzoyl)benzoic acid.
[0039] (3) In ...
Embodiment 2
[0043] Example 2 Preparation of compound (I)
[0044] Similar to Example 1, except that concentrated sulfuric acid was used as the reaction medium in step (3), and the yield was 27.6%.
Embodiment 3
[0045] Example 3 Preparation of compound (I)
[0046] Similar to Example 1, except that the reaction temperature in step (3) was 150°C, and the yield was 31.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com