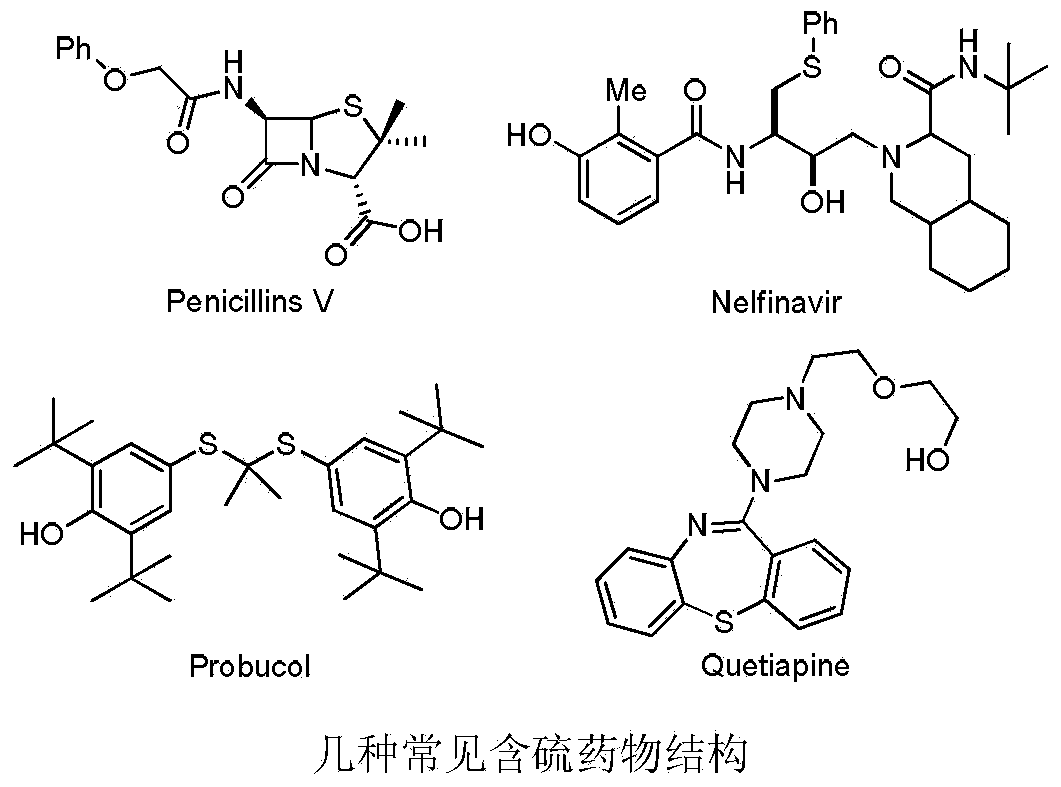
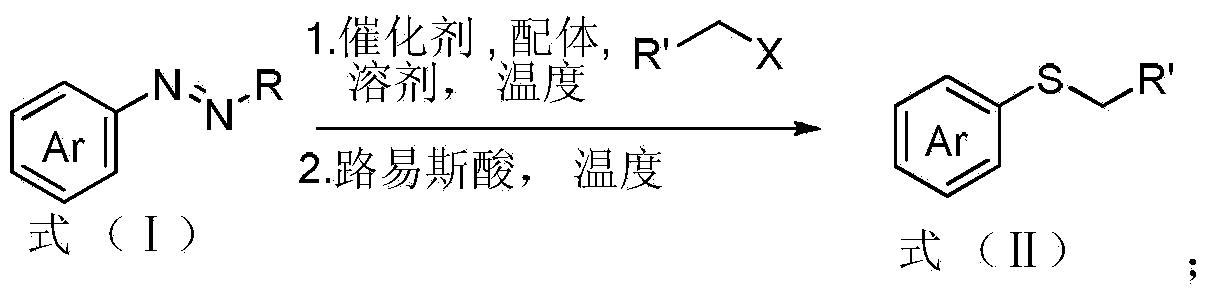
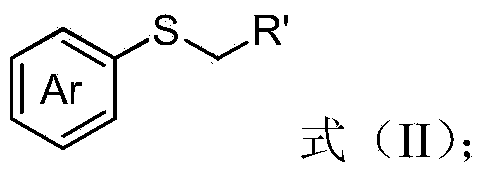
Aryl alkyl thioether compound and synthetic method thereof
A technology of aryl alkyl sulfide and synthesis method, which is applied in chemical instruments and methods, preparation of organic compounds, preparation of sulfide, etc., and can solve problems such as expensive catalyst, easy oxidation of vulcanizing reagent, pungent smell of metal catalyst, etc. , to achieve the effect of no irritating odor, high reaction efficiency and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Synthesis of benzyl (4-methoxyphenyl) sulfide:
[0040]
[0041] Catalyst CuI (3.8mg, 0.02mmol), ligand bipyridine L 1 (3.1mg, 0.02mmol), benzyl chloride (1mmol, 5equiv.) and Na 2 S 2 o 3 ·5H 2 O (248mg, 1mmol, 5equiv.) was added to the reaction tube, and then methanol / water (1mL / 1mL) was added as a reaction solvent, and stirred at a reaction temperature of 80°C for 2 hours. Then the temperature of the reaction system was lowered to 0°C in an ice bath, then triazene 1 (0.2 mmol, 1 equiv.) was added, and after stirring at room temperature for 15 minutes, BF was slowly added dropwise 3 ·Et 2 O (0.2 mmol, 1 equiv.). The reaction was then continued for 12 hours at room temperature. After the reaction was monitored by thin-layer chromatography, 5 mL of ethyl acetate was added to the reaction system and anhydrous sodium sulfate and anhydrous magnesium sulfate were added under continuous stirring. After stirring for five minutes, the mixture was filtered, and the fil...
Embodiment 2
[0043] Synthesis of benzyl (4-methoxyphenyl) sulfide:
[0044]
[0045] Catalyst CuCl (2.0mg, 0.02mmol), ligand bipyridine L 1 (3.1mg, 0.02mmol), benzyl chloride (1mmol, 5equiv.) and Na 2 S 2 o 3 ·5H 2 O (248mg, 1mmol, 5equiv.) was added to the reaction tube, and then methanol / water (1mL / 1mL) was added as a reaction solvent, and stirred at a reaction temperature of 80°C for 2 hours. Then the temperature of the reaction system was lowered to 0°C in an ice bath, then triazene 1 (0.2 mmol, 1 equiv.) was added, and after stirring at room temperature for 15 minutes, BF was slowly added dropwise 3 ·Et 2 O (0.2 mmol, 1 equiv.). The reaction was then continued for 12 hours at room temperature. After the reaction was monitored by thin-layer chromatography, 5 mL of ethyl acetate was added to the reaction system and anhydrous sodium sulfate and anhydrous magnesium sulfate were added under continuous stirring. After stirring for five minutes, the mixture was filtered, and the fi...
Embodiment 3
[0047] Synthesis of benzyl (4-methoxyphenyl) sulfide:
[0048]
[0049] Catalyst CuSO 4. 5H 2 O (2.0mg, 0.02mmol), ligand bipyridine L 1 (3.1mg, 0.02mmol), benzyl chloride (1mmol, 5equiv.) and Na 2 S 2 o 3 ·5H 2 O (248mg, 1mmol, 5equiv.) was added to the reaction tube, and then methanol / water (1mL / 1mL) was added as a reaction solvent, and stirred at a reaction temperature of 80°C for 2 hours. Then the temperature of the reaction system was lowered to 0°C in an ice bath, and triazene 1 (0.2 mmol, 1 equiv.) was added. After 15 minutes at room temperature, BF was slowly added dropwise. 3 ·Et 2 O (0.2 mmol, 1 equiv.). The reaction was then continued for 12 hours at room temperature. After the reaction was monitored by thin-layer chromatography, 5 mL of ethyl acetate was added to the reaction system and anhydrous sodium sulfate and anhydrous magnesium sulfate were added under continuous stirring. After stirring for five minutes, the mixture was filtered, and the filter ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com