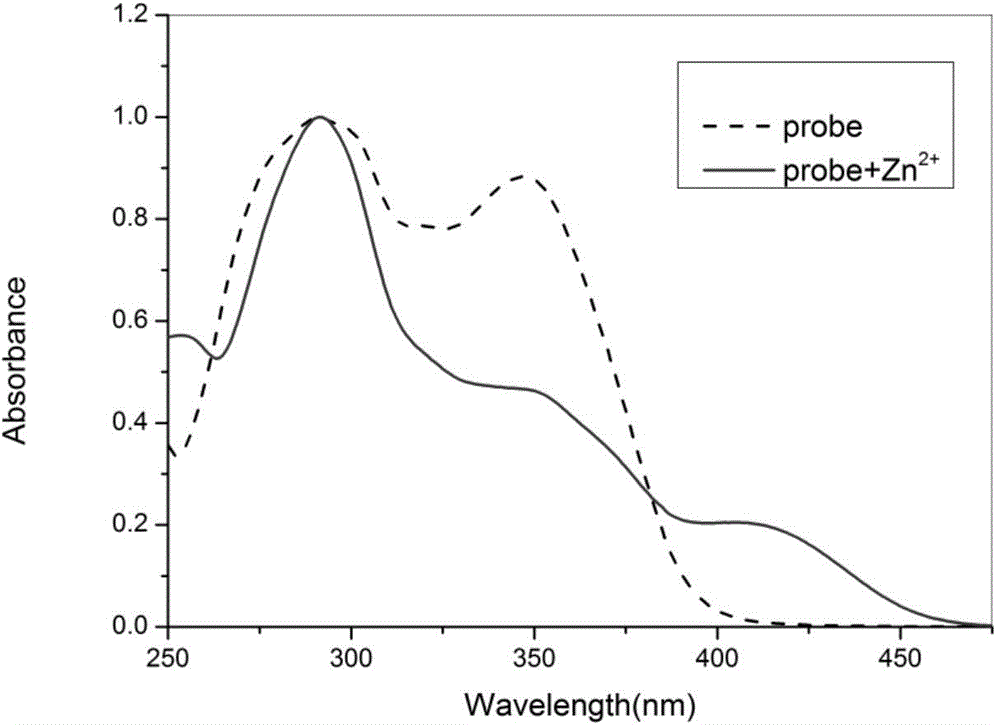
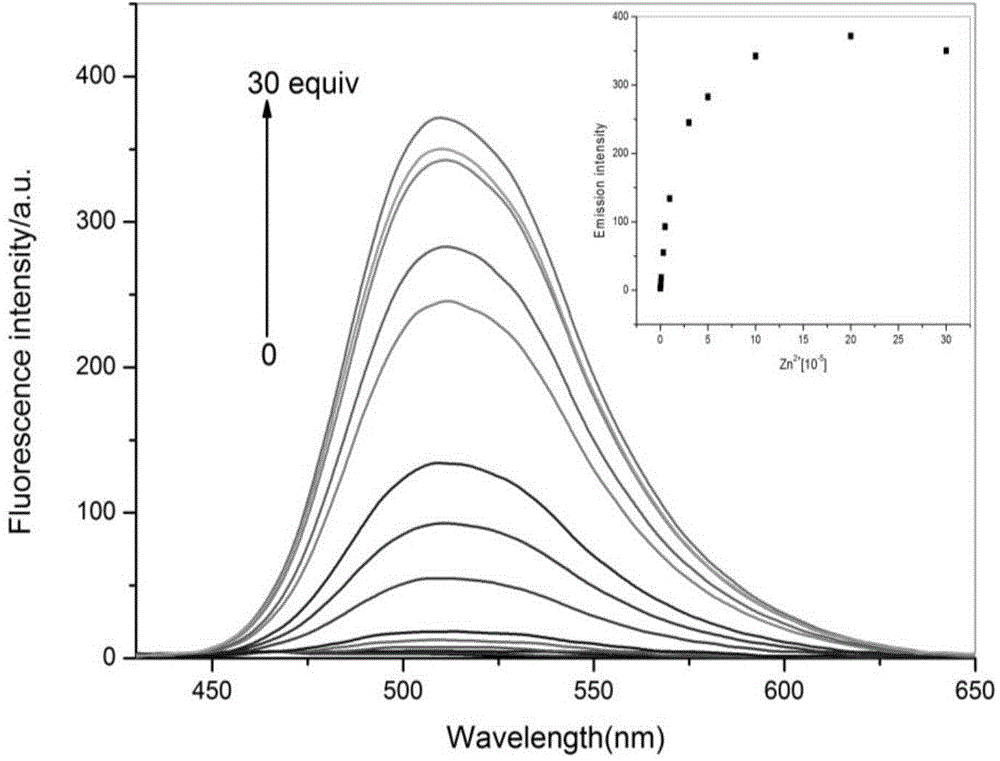
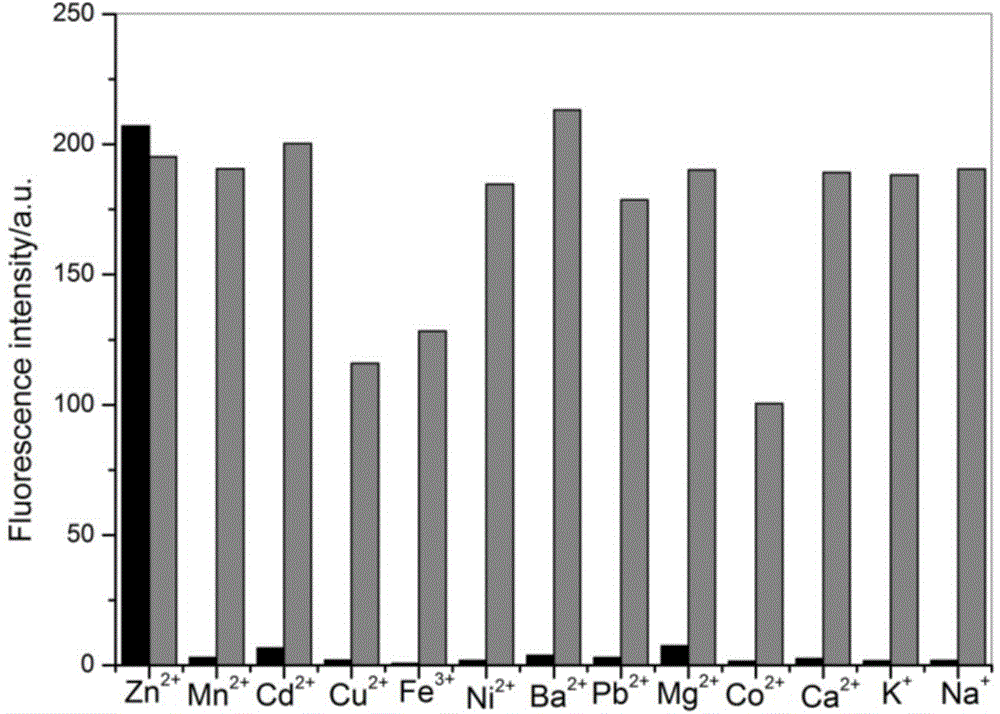
Functional active dye complexing with zinc ions and preparation method and application thereof
A reactive dye and functional technology, applied in reactive dyes, dyeing methods, azo dyes, etc., can solve the problems of low reuse rate, less functional research, single detection, etc. good use effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039]Dissolve 2g (13.2mmol) of methyl p-aminobenzoate in 20ml of absolute ethanol, slowly add 1ml (19.8mmol) of 98% hydrazine hydrate dropwise, reflux reaction for 3-4h, perform rotary evaporation on a rotary evaporator, and dissolve the solvent and excess Hydrazine hydrate was evaporated to obtain crude white solid p-aminobenzoic hydrazide, 1.6g (10.5mmol) p-aminobenzoic hydrazide was dissolved in absolute ethanol, and 1.3ml (11mmol) 98% salicylaldehyde was slowly added dropwise Put it into a three-necked bottle, heat and reflux for 1-2h, cool to obtain the crude product of salicylaldehyde-4-aminobenzoylhydrazine hydrazone, obtain the pure product through multiple recrystallizations from ethanol, filter to obtain golden yellow flaky crystals, and the yield 86%. FTIR(KBr):ν=3440cm -1 (-OH); 3041cm -1 (=CH)1720cm -1 (-C=O); 1625cm -1 (-C=N); 1289cm -1 (–C-N); 3041cm -1 (=CH); 1604cm -1 ,1571cm -1 Ar-H). 1 HNMR(400MHz,DMSO,δ / ppm),5.76(2H,s),6.96-7.03(2H,m),7.40-7.48(1H...
Embodiment 2
[0041] Dissolve salicylaldehyde-4-aminobenzoylhydrazide in acetone, make the temperature of the solution reach 0-5°C in an ice-water bath, then dissolve 99% cyanuric chloride with acetone, pour it into the dropping funnel and Slowly added dropwise to the three-necked flask. After the dropwise addition, stir for 1-2h, the same method NaHCO 3 The solution was added dropwise to prevent the solution from becoming acidic. The mixture was stirred at 0-5°C and reacted for 2-3h. After filtering, the product was washed several times with a small amount of acetone to obtain a functional reactive dye with a yield of 72.3%. FTIR(KBr):ν=3284cm -1 (-OH); 3199cm -1 (-NH-); 3055 (=CH); 1612cm -1 ,1538cm -1 (AR-H); 1514cm -1 ,1453cm -1 ,1388cm -1 (triazine);
[0042] 1 HNMR (400MHz, DMSO, δ / ppm): 5.75 (2H, s), 7.76-7.80 (4H, m), 7.94-8.01 (4H, m), 10.94 (1H, s), 11.32 (1H, s).
Embodiment 3
[0044] Dissolve 98% aminofluorene in 5 mL of THF and stir with a magnetic stirrer to dissolve it. And heated to 40-45°C in an oil bath, dissolved the condensed product with 15mL of THF, poured it into a dropping funnel, and slowly added it dropwise into a three-necked flask. After the dropwise addition, stir for 1h, the same method NaHCO 3 Add dropwise. After reacting for 3 hours, filter with suction to remove part of the solvent, and slowly drop the filtrate into 120 mL of petroleum ether for precipitation. A white solid was obtained. Put it into a vacuum drying oven at 50° C. to dry to obtain a dicondensed reactive functional dye. Yield 69%. mp: 237-238°C. FTIR (KBr) ν=3420cm -1 (-OH); 3304cm-1(-NH-); 3059cm -1 (=CH); 2955cm -1 (-CH2); 1707cm -1 (C=O); 1611cm-1, 1552cm -1 (Ar-H); 1516cm -1 ,1441cm -1 ,1417cm -1 (triazine).
[0045] 1H NMR (400MHz, DMSO, δ / ppm): 3.84 (2H, s), 3.91 (2H, br), 7.31-7.27 (2H, t, J = 15.01Hz), 7.40-7.36 (2H, t, J = 14.74 Hz), 7.58-7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com