Beta-amylase-trehalose synthase fused enzyme and expression gene and application thereof
A technology of trehalose synthase and gene expression, which is applied in the field of genetic engineering, can solve the problems of difficult separation of maltose and trehalose, difficult production conditions, and increased difficulty of separation and purification, so as to facilitate the control of process conditions and simplify the production process , Promote the effect of continuing to decompose starch
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] The construction of β-amylase expression vector, the steps are as follows:
[0047] The genomic DNA of Bacillus cereus was extracted, and the β-amylase gene fragment was amplified by PCR, and then the PCR amplified product was recovered from the gel, digested with restriction endonuclease BamHI / HindIII, and cloned into the same restricted Recombinant plasmid pET-BA was obtained by digesting the corresponding site of plasmid pET-28a with endonuclease BamHI / HindIII; the results are as follows: figure 1 shown.
[0048] Primer 1: 5'-CGCGGATCCATGAAAAATCAGTTTCAATATTGTTGTATTGTTGTTTTG;
[0049]Primer 2: 5'-CCCAAGCTTCCACCAACTTACATGAGAGGTAGTCTTCATTG;
[0050] The above PCR reaction system is shown in Table 1:
[0051] Table 1
[0052]
[0053] The above PCR reaction conditions: 94°C pre-denaturation for 5 min; 94°C denaturation for 30 sec, 54°C annealing for 30 sec, 72°C extension for 2 min, a total of 30 cycles; 72°C supplementary extension for 10 min.
Embodiment 2
[0055] To obtain engineering bacteria, the steps are as follows:
[0056] (1) E. coli BL21 (DE3) strain (purchased from Beijing Quanshijin Biotechnology Co., Ltd.) was inoculated on LB medium, and activated at 37°C overnight to obtain activated Escherichia coli;
[0057] (2) Pick a single colony of the activated Escherichia coli prepared in step (1) from the LB plate, inoculate it in 5 ml of fermentation medium, and culture it overnight with shaking at 37° C. until the late logarithmic growth period to obtain a bacterial suspension;
[0058] (3) Take the bacterial suspension prepared in step (2) and inoculate it into 100ml LB liquid medium at a ratio of 1:100 by volume, culture at 37°C, shake at 200r / min for 2.5h to OD 600 =0.5 (the early logarithmic phase), the Erlenmeyer flask was taken out and placed on ice for 15 minutes to prepare the bacterial solution;
[0059] (4) Pour the bacterial liquid from step (3) into a 50ml pre-cooled centrifuge tube under sterile conditions, ...
Embodiment 3
[0067] The construction of β-amylase and trehalose synthase fusion enzyme expression vector, the steps are as follows:
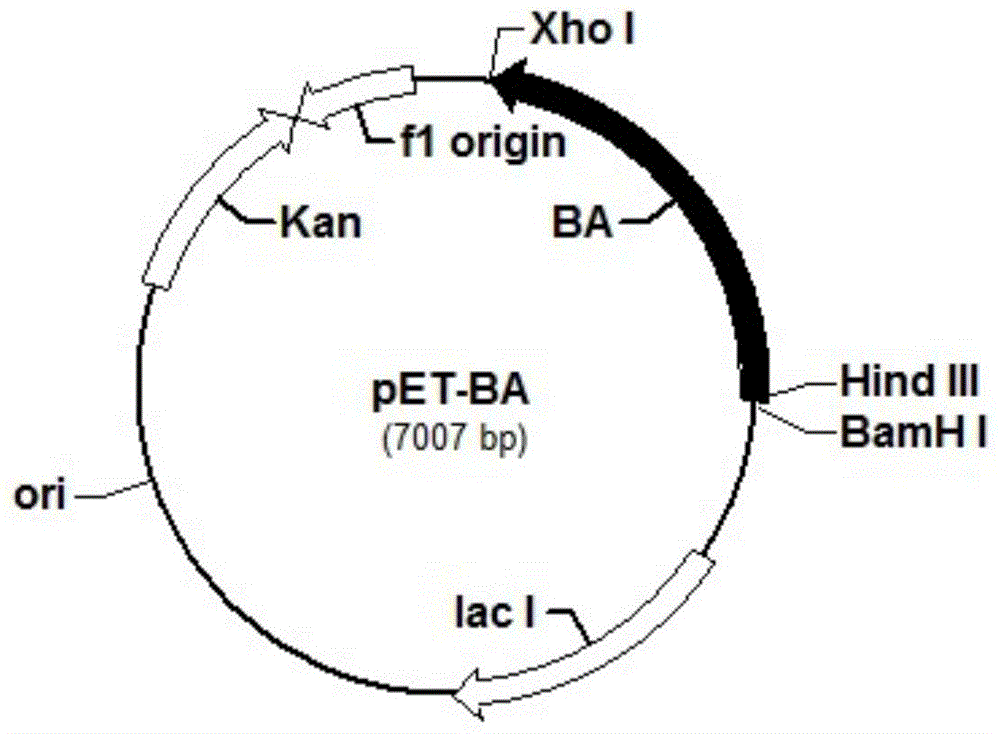
[0068] Using primer 3 and primer 4, the corresponding gene fragment of trehalose synthase in the genome of Pseudomonas putida was amplified in vitro (PCR). After restriction endonuclease digestion, it was cloned into the corresponding site of the vector pET-BA which was also digested with restriction endonucleases HindⅢ and XhoI to form the recombinant plasmid pET-BATS. Its structural map is as figure 2 shown. The PCR product is the corresponding gene fragment of trehalose synthase, the nucleotide sequence is shown in SEQ ID NO.5, and the amino acid sequence is shown in SEQ ID NO.6.
[0069] Primer 3: 5'-CCGCTCGAGTCAAACATGCCCGCTGCTGTTGAC,
[0070] Primer 4: 5'-CCCAAGCTTGAAGCTGCGGCAAAAGAAGCTGCGGCAAAAGAAGCTGCGGCAAAAATGACCCAGCCCGACCCGTCATAC;
[0071] The above-mentioned PCR reaction system is shown in Table 3:
[0072] table 3
[0073]
[0074] The ab...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com