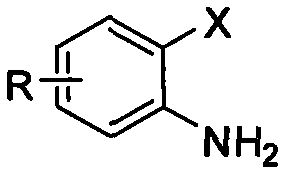
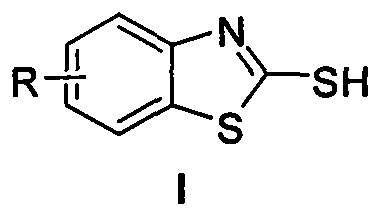
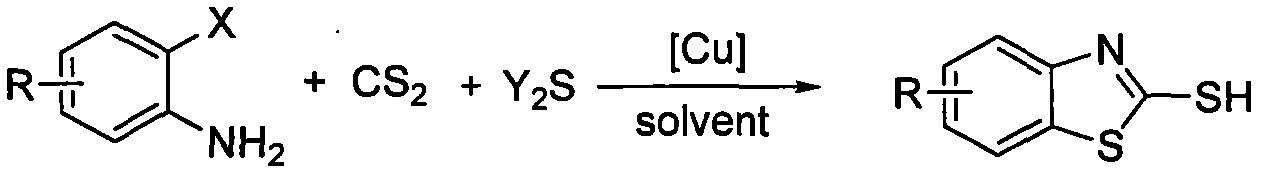2-mercapto benzothiazole derivative synthetic method with copper-catalyzed carbon disulfide
A synthesis method and carbon disulfide technology, applied in organic chemistry and other directions, can solve the problems of many by-products, long reaction time and low yield, etc., and achieve the effects of simple operation, cheap raw materials and high yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1, using o-iodoaniline as raw material to synthesize 2-mercaptobenzothiazole (reaction temperature 90°C)
[0018] Add 0.50mmol (0.1095g) of o-iodoaniline, 1.00mmol (0.2402g) of sodium sulfide nonahydrate and 0.05mmol (0.0095g) of cuprous iodide into the reaction test tube, and then add 2mL of solvent under an inert gas environment N,N-Dimethylformamide and 2.50mmol (0.1904g) of carbon disulfide were stirred at 90°C for 8 hours. After the reaction of o-iodoaniline was detected by TLC, the reaction solution was cooled to room temperature, and 3mL of 4N hydrochloric acid was added and stirred for 15min. , and then the reaction solution was extracted three times with dichloromethane, the organic phases were combined, dried over anhydrous magnesium sulfate for 2 hours, and then filtered to remove the desiccant, and finally the dichloromethane solvent was distilled off under reduced pressure to obtain a crude product. The crude product was separated by column chrom...
Embodiment 2
[0019] Embodiment 2, using o-iodoaniline as raw material to synthesize 2-mercaptobenzothiazole (reaction temperature 50°C)
[0020] Add 0.50mmol (0.1095g) of o-iodoaniline, 1.00mmol (0.2402g) of sodium sulfide nonahydrate and 0.05mmol (0.0095g) of cuprous iodide in the reaction test tube, and then add 2mL N , N-dimethylformamide and 2.50mmol (0.1904g) of carbon disulfide, stirred and reacted at 50°C for 8 hours, after the completion of the reaction of o-iodoaniline as detected by TLC, cooled the reaction solution to room temperature, added 3mL of 4N hydrochloric acid and stirred for 15min, Then the reaction solution was extracted three times with dichloromethane, the organic phases were combined, dried over anhydrous magnesium sulfate for 2 hours, and the desiccant was removed by filtration, and finally the dichloromethane solvent was distilled off under reduced pressure to obtain a crude product. The crude product was separated by column chromatography (200-300 mesh silica ge...
Embodiment 3
[0021] Example 3, Synthesis of 2-mercaptobenzothiazole with o-iodoaniline as raw material (reaction temperature 70°C)
[0022] Add 0.50mmol (0.1095g) of o-iodoaniline, 0.25mmol (0.0600g) of sodium sulfide nonahydrate and 0.05mmol (0.0095g) of cuprous iodide in the reaction test tube, and then add 2mL N , N-dimethylformamide and 1.50 mmol (0.1142 g) of carbon disulfide were stirred and reacted at 70°C for 8 hours. After the reaction of o-iodoaniline was detected by TLC, the reaction solution was cooled to room temperature, and 3 mL of 4N hydrochloric acid was added and stirred for 15 min. Then the reaction solution was extracted three times with dichloromethane, the organic phases were combined, dried over anhydrous magnesium sulfate for 2 hours, and the desiccant was removed by filtration, and finally the dichloromethane solvent was distilled off under reduced pressure to obtain a crude product. The crude product was separated by column chromatography (200-300 mesh silica gel)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com