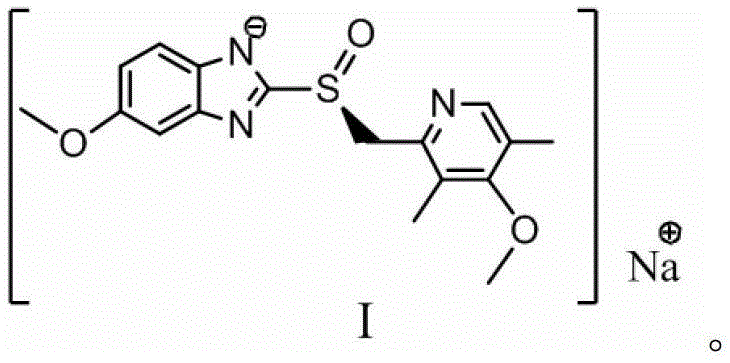
Preparation method for esomeprazole
A technology of esomeprazole and azole, which is applied in the field of drug synthesis, can solve the problems of heavy metal pollution, environmental harm, single chirality and the like, and achieves the effects of simple operation, low cost and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0092] C. the preparation method of esomeprazole
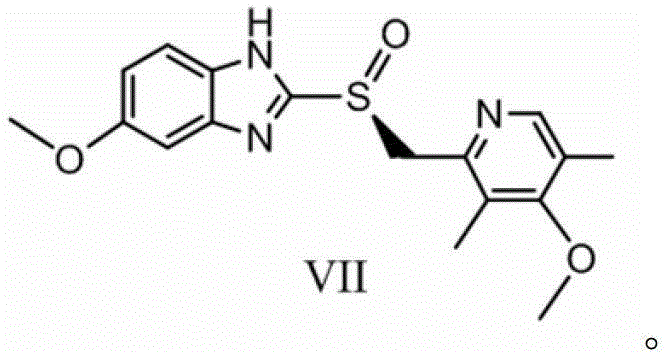
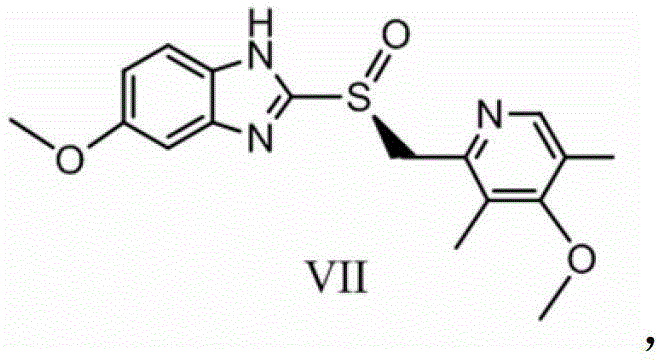
[0093] The present invention also provides a method for preparing esomeprazole, wherein said esomeprazole is shown in formula VII:
[0094]
[0095] The methods described include:
[0096] The following Grignard reaction occurs between the chiral compound shown in formula VI and the Grignard reagent shown in formula III, thereby obtaining the described esomeprazole:
[0097]
[0098] Wherein, M is magnesium or zinc, preferably magnesium; X is halogen, preferably chlorine, bromine or iodine, more preferably chlorine.
[0099] Preferably, the molar ratio of intermediate VI to intermediate III Grignard reagent of the Grignard reaction is 1:(1-1.2); more preferably 1:(1-1.1). Too high or too low a molar ratio will lead to excess raw materials.
[0100]Preferably, the Grignard reaction is carried out in an aprotic solvent, and the aprotic solvent is preferably tetrahydrofuran, methyl tert-butyl ether, toluene or xylene, pr...
Embodiment 1
[0149] Embodiment 1: the preparation of the Grignard reagent shown in formula III:
[0150] Keep warm at about 20°C, replace the reaction system with nitrogen, add 150ml of tetrahydrofuran (hereinafter referred to as THF) and 68.0g (2.80mol) of magnesium chips (after pretreatment), and 12.7g (0.05mol) of iodine into a 1000ml reaction bottle, and stir for 1 Hours, the temperature was raised to 30°C, and at the same time, 464.3g (2.50mol) and 12.7g (0.05mol) of 2-(chloromethyl)-4-methoxyl-3,5-lutidine dissolved in 150ml THF were slowly added dropwise. ) iodine mixture, after dropping, keep warm at 30°C for 5 hours, cool to 20°C to 25°C, and set aside.
[0151] Among them, the pretreatment method of magnesium chips is as follows: stir and wash with 5% hydrochloric acid for 30 minutes, quickly filter and rinse with acetone (minimize the time of contact with air), and use it immediately after vacuum drying.
Embodiment 2
[0152] Embodiment 2: the preparation of the Grignard reagent shown in formula III:
[0153] Keep warm at about 20°C, replace the reaction system with nitrogen, add 150ml of tetrahydrofuran (hereinafter referred to as THF) and 183.1g (2.80mol) of zinc dust (after pretreatment), and 12.7g (0.05mol) of iodine into a 1000ml reaction bottle, and stir for 1 Hours, the temperature was raised to 30°C, and at the same time, 464.3g (2.50mol) and 12.7g (0.05mol) of 2-(chloromethyl)-4-methoxyl-3,5-lutidine dissolved in 150ml THF were slowly added dropwise. ) iodine mixture, after dropping, keep warm at 30°C for 8 hours, cool to 20°C to 25°C, and set aside.
[0154] Among them, the pretreatment method of zinc shavings is as follows: stir and wash with 5% hydrochloric acid for 30 minutes, quickly filter and rinse with acetone (minimize the time of contact with air), and use it immediately after vacuum drying.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com