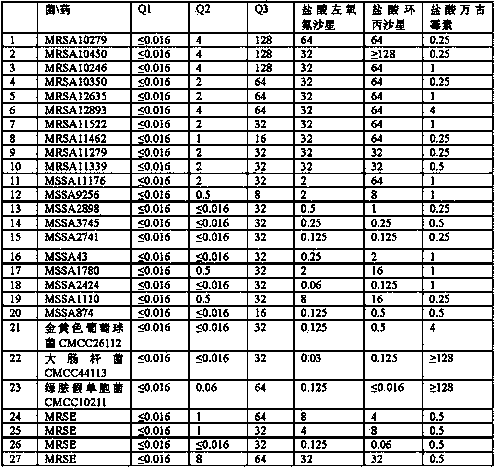
Tricyclic quinolone derivative, preparation method and application thereof
A quinolone, quinolone technology, applied in the field of medicinal chemistry and chemotherapeutics, can solve problems such as adverse drug reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Example 1: (S)-9-fluoro-10-(4-hydroxypiperidin-1-yl)-3-methyl-7-carbonyl-3,7-dihydro-2H-[1,4]oxa Preparation of oxazine[2,3,4-ij]quinoline-6-carboxylic acid L-arginine salt tetrahydrate (Q1)
[0071]
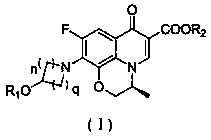
[0072] Step (a): Add boric acid (48.00g), acetic anhydride (220ml) and zinc chloride (0.88g) into a dry three-necked flask, stir at room temperature for 30min, then add ( S )-9,10-difluoro-3-methyl-7-carbonyl-3,7-dihydro-2H-[1,4]oxazine[2,3,4- ij ] Ethyl quinoline-6-carboxylate (1) (80.00g) was added to the above solution, reacted at 60°C for 16h, concentrated under reduced pressure, slowly added dichloromethane (2200ml) to the concentrate, and washed with saturated NaHCO 3 Solution washing (2×1400ml), liquid separation, organic layer was washed with NaCl solution, anhydrous NaCl 2 SO 4 Dry, filter, and concentrate the filtrate to obtain a solid, then add 900ml of anhydrous ether, stir for 30min, filter, and dry the solid in vacuum to obtain bis(acetyl- O )[(3 S ...
Embodiment 2
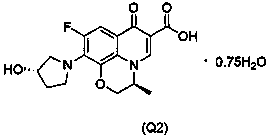
[0083] Example 2: (S)-9-fluoro-10-((S)-3-hydroxypyrrol-1-yl)-3-methyl-7-carbonyl-3,7-dihydro-2H-[1, 4] Preparation of oxazine[2,3,4-ij]quinoline-6-carboxylic acid 0.75 hydrate (Q2)
[0084]
[0085] Step (a) is the same as step (a) of Example 1.
[0086] Step (b): Add bis(acetyl- O )[(3 S )-9,10-difluoro-2,3-dihydro-3-methyl-7-dihydro-7 H -pyridin[1,2,3- de ][1,4]Benzoxazine-6-carboxylate- O 6 , O 7 ] Boron(2) (8.00g), ( S )-3-hydroxypyrrole hydrochloride (3.63g) and triethylamine (5.94g), then add acetonitrile 120ml, react at 75°C for 3h, concentrate to dryness under reduced pressure, slowly add dichloromethane (150ml) and water (150ml), separated, and the organic layer was washed with NaCl solution, anhydrous Na 2 SO 4 Dry, filter, and concentrate the filtrate to obtain 9.29 g of a yellow solid. The yield of the crude product is quantitative, and it is directly put into step (c) for reaction.
[0087] Step (c): The solid (9.29g) obtained in the above step (b)...
Embodiment 3
[0094] Embodiment 3: ( S )-9-fluoro-10-(( S )-3-hydroxypyrrol-1-yl)-3-methyl-7-carbonyl-3,7-dihydro-2H-[1,4]oxazine[2,3,4- ij ] Preparation of methyl quinoline-6-carboxylate (Q3)
[0095]
[0096] Steps (a), (b) and (c) are the same as Steps (a), (b) and (c) of Example 2.
[0097] Step (d): add ( S )-9-fluoro-10-(( S )-3-hydroxypyrrol-1-yl)-3-methyl-7-carbonyl-3,7-dihydro-2H-[1,4]oxazine[2,3,4- ij ]quinoline-6-carboxylic acid (8.00g), K 2 CO 3 (6.36g) and DMF (46ml), stir well and add CH 3 I (4.90g), then heated to 50°C for 8h, cooled to room temperature, added ethyl acetate (100ml) and water (50ml), separated, the aqueous phase was extracted with ethyl acetate (100ml), separated, combined organic phase, the organic phase was washed with water (200ml), washed with saturated brine (200ml), Na 2 SO 4 After drying, filtering, and concentrating the filtrate to obtain a crude product, the crude product was recrystallized in ethanol to obtain 6.52 g of the product (Q3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com