Method for preparing highly concentrated fibrinogen solution and method for preparing fibrin sealant by using thereof
A fibrinogen, highly concentrated technology, used in the preparation of highly concentrated fibrinogen solution, fibrin sealant product field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Example 1: Preparation of highly concentrated fibrinogen solution
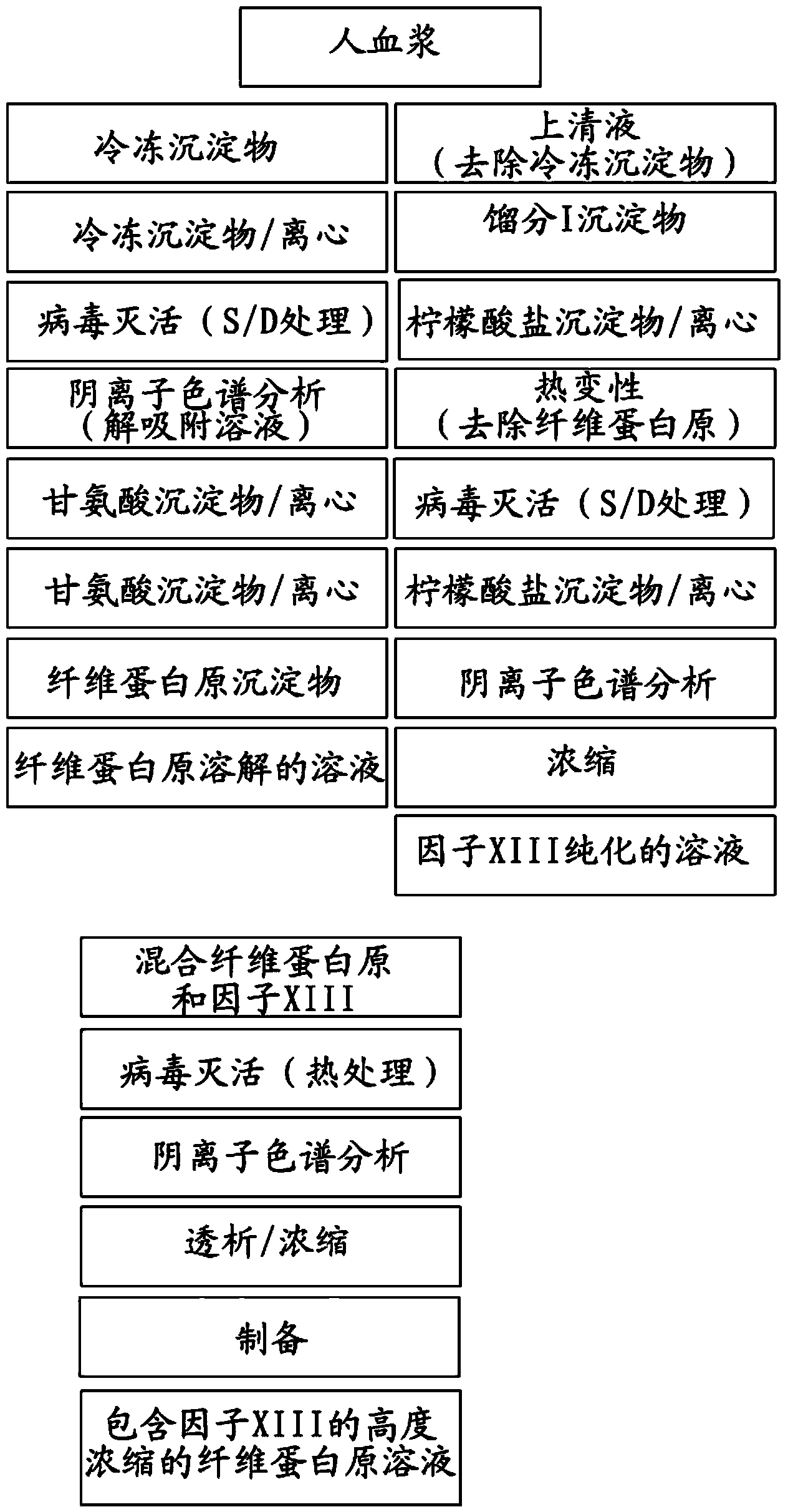
[0076] The low concentrated fibrinogen solution and factor XIII in the present invention are isolated and prepared from human plasma. First, after separation of the cryoprecipitate from the human plasma, fibrin was prepared using cryoprecipitation, virus inactivation (using a solvent / detergent (S / D) treatment method), anion chromatography, glycine precipitation The original sediment (see figure 1 ).
[0077] After removal of the cryoprecipitate from human plasma, after the preparation of Factor XIII from fraction I obtained by ethanol precipitation by the citrate precipitation process, the S / D treatment process, the anion chromatography process and the concentration process, the fiber The protein precipitate is dissolved and mixed, and then heat-treated (10 hours at 60°C), which is a virus inactivation process, anion chromatography process, and dialysis concentration process, so that the low-level ...
Embodiment 2
[0085] Example 2: Stability of fibrin sealant component 1 according to temperature storage conditions
[0086] A fibrin sealant component 1 was prepared by mixing 10 mg / mL albumin, 1000 KIU / mL aprotinin, and Tween 80 with the highly concentrated fibrinogen solution prepared according to Example 1.
[0087] The prepared fibrin sealant component 1 was sterilized by filtration using a 0.2 μm filter to be filled in a 1 mL syringe, thus confirming stability according to storage conditions. The fibrin sealant component 1 used in this experiment contained 88 mg / mL of fibrinogen and 65 IU / mL of factor XIII.
[0088] Stability was assessed by measuring the time to clotted fibrinogen protein reduced by 10% as shelf life.
[0089] As a result, it was observed that the fibrin sealant component 1 comprising Factor XIII according to the invention was stable for at least 24 months at -18°C and up to 6 months at 25°C (see Table 3).
[0090] Table 3. Stability of fibrin sealant component 1...
Embodiment 3
[0092] Embodiment 3: the preparation of fibrin sealant product
[0093] The fibrin sealant component 1 prepared in Example 2 was supplied with fibrin sealant component 2 containing thrombin and calcium chloride filled in separate containers to prepare a fibrin sealant product, and finally, the two The two solutions are mixed with each other so that fibrin polymers are formed and can be used for medical purposes etc. In the fibrin sealant component 2 filled in a separate container, 500 IU / mL of thrombin and 40 mM of calcium chloride were contained, and 40 mg / mL of albumin may be additionally added.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com