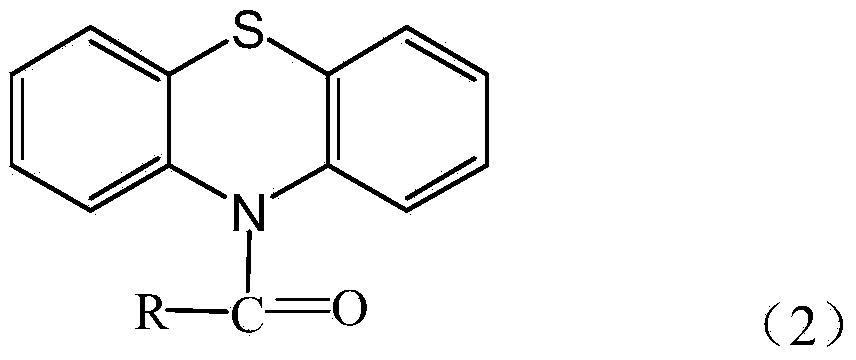
Method for preparing N-acyl phenothiazine
A technology of acyl phenothiazine and phenothiazine, which is applied in the field of preparing N-acyl phenothiazine, can solve the problems of long reaction process time, troublesome post-processing, environmental pollution, etc., and achieve short reaction time, small environmental pollution, and post-processing Handle simple effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Add 0.0055mol benzoic acid and 0.0055mol phosphorus oxychloride to a dry mortar, grind for 0.5h at room temperature until the grinding is uniform, then add 0.005mol phenothiazine, continue grinding, monitor the reaction with TLC, until the phenothiazine is completely reacted At this time, the raw material point of phenothiazine disappeared, and the grinding was stopped to obtain the product, wherein the developer used for TLC monitoring was a mixed solution of ethyl acetate and petroleum ether with a volume ratio of 1:3, and then the product was allowed to stand at room temperature for 1h ; Then wash with suction and filter, dry the filter cake at room temperature for 24 hours and then recrystallize with water to obtain N-acylphenylphenothiazine. The yield was 93.1%. The melting point is 150-154°C.
[0028] IR(KBr,cm -1 ):3089cm -1 ,3056cm -1 (Unsaturated C-H); 1671cm -1 (amide); 1596cm -1 , 1502cm -1 , 1472cm -1 (benzene ring vibration absorption peak); 1256cm ...
Embodiment 2
[0030] Add 0.0055mol m-toluic acid and 0.0055mol phosphorus oxychloride to a dry mortar, grind for 1 hour at room temperature until the grinding is uniform, then add 0.005mol phenothiazine, continue grinding, monitor the reaction with TLC, until the phenothiazine Reaction is complete, and now the raw material point of phenothiazine disappears, and the grinding is stopped to obtain the product, wherein the developing agent used for TLC monitoring is a mixed solution of ethyl acetate and sherwood oil with a volume ratio of 1:3, and then the product is statically placed at room temperature. Set aside for 0.5h; then wash with water and filter with suction, dry the filter cake at room temperature for 22h and then recrystallize with water to obtain N-acyl m-methylphenylphenothiazine. The yield was 94.4%. The melting point is 110-112°C.
[0031] IR(KBr,cm -1 ):3098cm -1 ,3054cm -1 (Unsaturated C-H); 2994cm -1 ,2911cm -1 (saturated C-H); 1656cm -1 (amide); 1595cm -1 , 1500cm ...
Embodiment 3
[0033] Add 0.005mol p-chlorobenzoic acid and 0.0055mol phosphorus oxychloride to a dry mortar, grind for 0.8h at room temperature until the grinding is uniform, then add 0.005mol phenothiazine, continue grinding, monitor the reaction with TLC, until the phenothiazine Reaction is complete, and now the raw material point of phenothiazine disappears, and the grinding is stopped to obtain the product, wherein the developing agent used for TLC monitoring is a mixed solution of ethyl acetate and sherwood oil with a volume ratio of 1:3, and then the product is statically placed at room temperature. Set aside for 0.8h; then wash with water and filter with suction, dry the filter cake at room temperature for 23h and then recrystallize with water to obtain N-acyl p-chlorophenylphenothiazine. The yield was 92.6%. The melting point is 134-139°C.
[0034] IR(KBr,cm -1 ):3089cm -1 , 3024cm -1 (Unsaturated C-H); 1654cm -1 (amide); 1598cm -1 , 1502cm -1 , 1459cm -1 (benzene ring vibra...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com