Patents
Literature
99results about How to "Short corrosion time" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Corrosive agent for displaying silicon steel coagulation tissue and its preparation method
InactiveCN101144762AReactive and restrictiveAvoid irritation hazardsPreparing sample for investigationChemical agentPrecipitation
The invention provides a corrosive for the revealing of the silicon steel solidification structure. The invention has the component and blending ratio of the corrosive that 0.9 to 1.2 gram of a picronitric acid and / or 0.9 to 1.2 gram of a stannous acid, 10 to 20 gram of a ferric chloride, 20 to 50 milliliter of an absolute ethyl alcohol, 15 to 18 milliliter of a hydrochloric acid, 0.2 to 0.5 gram of a copper chloride, 0.2 to 0.5 gram of a magnesium chloride and 80 to 100 milliliter of a distilled water. The invention has the dosing method of the corrosive that the picronitric acid and / or the stannous acid and the ferric chloride are put into a container; the distilled water is divided into two shares, and one share is added into a medicine container; the absolute ethyl alcohol is divided into two shares, one share is added into an aqueous solution which is mixed with medicine, and the other share is added into the distilled water; the hydrochloric acid is added into the ethyl alcohol aqueous solution; the aqueous solution containing ethyl alcohol and hydrochloric acid is poured into the aqueous solution which is mixed with medicine, and the copper chloride and the magnesium chloride are added in. The chemical agent which is used in the corrosive reciprocally reacts mutually and has the strong conditionality, the efficiency of the core corrosive is exerted without forming a surface precipitation membrane, and a clear tree like crystal shape can be directly observed; the corroding time of the corrosive is short, and is suitable for a large size continuous casting semi finished metal; the dosing method of the corrosive is scientific, and the operating procedure is simple, convenient and easy to operate.
Owner:ANGANG STEEL CO LTD
Metallographic corrosion method for martensitic precipitation hardening stainless steel crystal boundary
InactiveCN102443803ADoes not affect observationNo heating requiredPreparing sample for investigationMartensiteHigh-temperature corrosion
The invention discloses a metallographic corrosion method for a martensitic precipitation hardening stainless steel crystal boundary, wherein the stainless steel is 05Cr17Ni4Cu4Nb martensitic precipitation hardening stainless steel. The method comprises the following steps: 1, coarsely grinding, finely grinding, polishing, cleaning and drying a metallographic sample to obtain a shine scratch-freepolished surface; and 2, picking up a corrosive agent, wiping the polished surface for 15-20s, flushing, and drying, wherein the corrosive agent is prepared by pouring 2-3ml of concentrated hydrochloric acid into 30ml of concentrated nitric acid, uniformly stirring and allowing the obtained solution to stand for above 5min. According to the invention, the 05Cr17Ni4Cu4Nb martensitic precipitation hardening stainless steel crystal boundary can be intelligibly and completely displayed, and the color and the morphology of the tissue have no influences on the observation of the contour of the crystal boundary. The method has the following advantages: the crystal boundary display effect is excellent; normal temperature corrosion and no need of heating are realized; and the corrosion time is short, and only 15-20 seconds are needed.
Owner:725TH RES INST OF CHINA SHIPBUILDING INDAL CORP
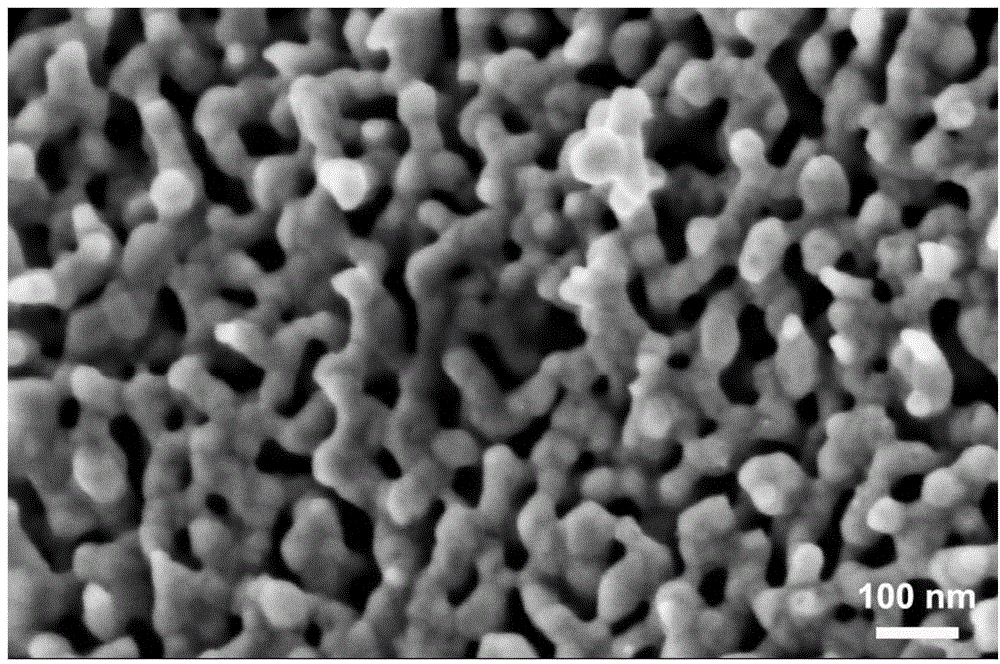
Method for preparing nanometer porous copper powder
The invention discloses a method for preparing nanometer porous copper powder. The method comprises the following steps of: weighing pure copper powder and pure aluminum powder in a ratio of Al-Cu alloy ingredients; adding the pure copper powder, pure aluminum powder and grinding balls into a ball-milling tank, adding a grinding aid, and performing mechanical alloying treatment in a ball-milling machine to obtain Al-Cu alloy powder; and performing dealloying on the obtained Al-Cu alloy powder in alkaline solution, and washing a sample until the sample is neutral to obtain the nanometer porouscopper powder. Precursor alloy is prepared by a mechanical alloying method, and a process is simple; precursor alloy powder has small sizes, so the corrosion time is shortened greatly (within 1 hour generally), and the production efficiency is improved greatly; and the hole size of the prepared nanometer porous copper is only between 15 and 80 nanometers and is reduced greatly, the porous copper is the powdery sample, and a specific surface area is improved greatly.
Owner:UNIV OF JINAN
Elastic compound metal heat interface material and preparation method thereof
The invention belongs to the technical field of a heat interface material and discloses an elastic compound metal heat interface material. The elastic compound metal heat interface material is formed by compounding indium and a through-hole porous metal sheet, wherein the indium is filled in the through-hole porous metal sheet and covers the upper and lower surfaces of the through-hole porous metal sheet. The invention also discloses a preparation method of the elastic compound metal heat interface material. The method comprises the following steps: preparing the through-hole porous metal sheet at first; adding a proper amount of indium; heating for smelting indium so that smelted indium fills in the through-hole porous metal sheet and covers the upper and lower surfaces of the through-hole porous metal sheet; and finally cooling, thereby obtaining the elastic compound metal heat interface material. The two surfaces of the through-hole porous metal sheet are both through-hole porous metal and the middle core of the through-hole porous metal sheet is a metal solid. The compound material of the through-hole porous metal sheet and indium has higher elasticity and flexibility in the direction vertical to a mounting surface, so that the compound material can be quickly filled in bigger gaps caused by heat sink and an uneven silicon surface, the interface can be completely filled and the heat resistance of the interface can be kept.
Owner:SOUTHEAST UNIV
Corrosion liquid and corrosion method for ultralow steel original austenite crystal boundary
InactiveCN104374627AImprove efficiencyReduce energy consumptionPreparing sample for investigationCarbon steelMicroscopic observation
The invention discloses corrosion liquid and a corrosion method for an ultralow steel original austenite crystal boundary. The corrosion liquid comprises the following components in parts by volume: 19-21 parts of supersaturated picric acid, 1.8-2.2 parts of a detergent and 0.8-1.2 parts of hydrochloric acid, wherein the concentration of the hydrochloric acid is 37%, and the detergent is cleanser essence. The corrosion method for the ultralow steel original austenite crystal boundary by using the corrosion liquid comprises the steps of (1) sampling ultralow carbon steel by cutting and then carrying out metallographic grinding and polishing on the sampled ultralow carbon steel; (2) soaking the polished ultralow carbon steel into the corrosion liquid for corrosion; (3) by the end of the corrosion process, scrubbing the ultralow carbon steel with a saturated oxalic acid solution, and blow-drying; (4) after blow-drying, observing the ultralow carbon steel by a metallographic microscope to obtain an original austenite structure figure, wherein the soaking time is 180-220 seconds in the step (2). By the corrosion liquid and the corrosion method, the time is shortened, the reagent is saved, and the clear original austenite crystal boundary can be obtained.
Owner:苏州华碧微科检测技术有限公司
Rapid metallographic determination method for grain size of GCr15 steel bearing assembly
InactiveCN103344532AEasy to operateAccelerated corrosionPreparing sample for investigationParticle size analysisMetallurgyImaging analysis
The invention belongs to the technical field of bearing detection. The invention relates to a rapid metallographic determination method for of grain size of a GCr15 steel bearing assembly. The rapid metallographic determination method comprises the following steps of 1), preparing an etching solution; 2), etching a sample of the bearing assembly; 3), determining the grain size: shooting a metallograph of a grain boundary profile by using an optical microscope, then determining the grain size of the metallograph by using image analysis software and in reference to steel size determination standards of an average grain size of the steel by an intercept method. The clear grain boundary profile can be obtained by using a rapid and easily operational chemical etching method; and efficient and accurate determination for the GCr15 steel bearing assembly can be realized by combining the metallographic determination method for the grain size.
Owner:WUHAN UNIV OF TECH
Nanometer porous silver alloy material and preparation method thereof
InactiveCN104674045AUniform tissueUniform three-dimensional porous structureSolid solutionControllability
The invention discloses a nanometer porous silver alloy material and a preparation method thereof. Specifically, amorphous alloy is taken as a precursor; elements, excluding noble metals such as Ag, Pt, Pd,Au, Ru, Rh, Os and Ir in the alloy, are corroded by a dealloying method to form a nanometer porous structure; a lace is elemental silver or silver alloy (a solid solution, the solute atom is noble metals such as Pt, Pd, Au, Ru, Rh, Os and Ir); the feature size of both the pores and the lace is smaller than 50 nanometers. The nanometer porous silver alloy material prepared by the method disclosed by the invention has self-sustaining property and structural controllability and can maintain the structural integrity and self-sustaining property after 70% of mass is lost. The preparation method and a detection method are simple; the obtained material can be widely applied to various catalytic reactions.
Owner:BEIHANG UNIV
Transmissive metal grating and preparation method thereof
InactiveCN1790067AReduce processEasy to manufactureSemiconductor/solid-state device manufacturingDiffraction gratingsTransmittanceCopper
The invention discloses a transmission type metal raster, which comprises the following parts: metal base plate, raster bright fringe and raster dark fringe, wherein the raster bright fringe is the gap of metal base plate with 100 percent light transmittance ratio; the dark fringe is metal base plate itself without light transmission; the metal base plate is stainless steel, copper and aluminum film or metal ruler. The transmission type metal raster making method comprises the following steps: washing the metal base plate surface; coating even thickness photoresist on two sides of base plate; removing the dilute agent of photoresist by drying; placing the base plate in two same raster motherboards to align then exposing; developing through the developing liquid; corroding the toast hard film in the FeCl3 liquid; removing the photoresist; cleaning the base plate surface.
Owner:INST OF OPTICS & ELECTRONICS - CHINESE ACAD OF SCI
Dislocation identification method of silicon carbide crystal
PendingCN111238910AShort corrosion timeWell representedPreparing sample for investigationOptically investigating flaws/contaminationCarbide siliconErosion corrosion
The invention provides a dislocation identification method of a silicon carbide crystal. The method comprises the following steps: (1) corroding the silicon carbide crystal in an alkaline corrosive agent; and (2) after the corrosion is completed, observing the morphology of a dislocation corrosion pit so as to identify blade dislocation, screw dislocation and base plane dislocation in the siliconcarbide crystal, wherein the silicon carbide crystal comprises high-purity silicon carbide and nitrogen-doped silicon carbide, the corrosion time of the high-purity silicon carbide is 5-7 minutes, andthe corrosion time of the nitrogen-doped silicon carbide is 7-9 minutes. According to the method, the corrosion time of alkaline corrosion is optimized, and the corrosion time of high-purity siliconcarbide and nitrogen-doped silicon carbide is controlled to be less than 10 minutes so that three dislocations including edge dislocations, screw dislocations and base plane dislocations in the high-purity silicon carbide or the nitrogen-doped silicon carbide can be accurately distinguished; the corrosion time is short; and three dislocations in the crystal can be accurately identified.
Owner:SICC CO LTD
Frequency conversion bore distribution method for low-voltage anodic foil for aluminum electrolytic capacitor
InactiveCN101235533AReduce dispersion rateHigh hole densityElectrolytic capacitorsElectrolysisLow voltage
A method for conversing frequency and distributing holes used in low voltage anode foil of aluminum electrolytic capacitors relates to aluminum electrolytic capacitors, in particular relates to a method which can realize conversing frequency and distributing holes used in low voltage anode foil of the aluminum electrolytic capacitors with high specific volume aluminum electrolytic capacitors and provides a method for conversing frequency and distributing holes used in low voltage anode foil of aluminum electrolytic capacitors, wherein the method comprises the following steps: carrying out conventional oil removal to the aluminum foil, cleaning, and then carrying out hole distribution electrolysis to the aluminum foil, wherein the electrolyte is provided with 0.8-3.8N hydrochloric acid, 0.01-0.5N vitriol oil and 0.1-1.2N alchlor, frequency of the simple-sinusoidal alternating current which is adopted by the electrolysis is 5-80Hz, current density is 0.20-0.37A / cm2, obtaining the etched foil after carrying out conventional hole enlarging, obtaining the aluminum electrolytic capacitors used in low voltage anode foil after carrying out the conventional forming to the etched foil.
Owner:XIAMEN UNIV +1
Method for fast displaying original austenite grains of maraging steel containing Cr
InactiveCN103411814AEasy to operateShort corrosion timePreparing sample for investigationCorrosionRoom temperature
The invention relates to a method for fast displaying original austenite grains of maraging steel containing Cr. The method is characterized by comprising the following steps: a, preparing a sample, wherein an aging state sample is subjected to grinding and polishing; b, configuring a corrodent, wherein 10 ml distilled water is poured in a utensil, 10 ml hydrochloric acid and 1-2 ml nitric acid are added into the distilled water, stirring by a glass rod is performed, still standing is carried out, the weight percentage of the hydrochloric acid is 36%, and the weight percentage of the nitric acid is 68%; c, performing sample corrosion, wherein absorbent cotton is placed at the bottom of the utensil, the polished sample is placed in the corrodent for corrosion, the erosion surface of the sample is downward, the erosion time is 20-30 s, when the polished surface of the sample becomes gray, the sample is taken out, cleaned and dried, and the austenite grains are observed under a microscope. The method has the advantages that heating is eliminated, and the operation can be carried out at room temperature; the operation is simple, and the corrosion time is short; the repeatability is better.
Owner:INNER MONGOLIA BAOTOU STEEL UNION
Metallographic erosion method of nickel-aluminum bronze crystal boundary
InactiveCN105352779AThe grain boundary is clear and completeNo heating requiredPreparing sample for investigationAlcoholGranularity
A metallographic erosion method of a nickel-aluminum bronze crystal boundary is provided. The method includes polishing the nickel-aluminum bronze by waterproof abrasive papers with granularity of 80, 280, 400, 600 and 800 in order, polishing the nickel-aluminum bronze by a lint and a 1 [mu]m diamond polishing slurry in order, adding nitric acid into hydrochloric acid, stirring the mixture uniformly, allowing the mixture to stand for 2 min to prepare a corrosive agent which has a preparation ratio according to every 30 ml hydrochloric acid in every 3-5 ml nitric acid, dipping the corrosive agent by an absorbent cotton clamped by a plastic tweezer, wiping the surface of the nickel-aluminum bronze for 5-15 s until the color of the surface of the nickel-aluminum bronze turns grey, washing the surface of the nickel-aluminum bronze by clear water, flushing the surface of the nickel-aluminum bronze by absolute ethyl alcohol, blow-drying the surface of the nickel-aluminum bronze by a electric hair drier, and putting the blow-dried nickel-aluminum bronze by the electric hair drier to a metallographic microscope to obtain a crystal boundary photograph of the nickel-aluminum bronze. The etching time is only 5-10 s, the crystal boundary not a K phase of the nickel-aluminum bronze can be preferentially etched, and the crystal boundary of the nickel-aluminum bronze is clear and intact.
Owner:725TH RES INST OF CHINA SHIPBUILDING INDAL CORP
Polycrystalline silicon etching solution in solar cell and polycrystalline silicon etching process
InactiveCN102330156AShort corrosion timeIncrease daily outputAfter-treatment detailsPolycrystalline siliconSolar cell
The invention relates to a polycrystalline silicon etching wafer solution in a solar cell. The solution is characterized by comprising the following components in parts by weight: 6 to 7 parts of hydrofluoric acid, 64 to 68 parts of nitric acid, and 26 to 28 parts of deionized water. An etching process of the polycrystalline silicon in the solar cell is characterized by comprising the following steps: injecting the deionized water into an etching tank; slowly pouring the hydrofluoric acid into the etching tank along the etching tank wall; slowly pouring the nitric acid into the etching tank along the etching tank wall; starting a cooling system of the etching tank to circularly cool a liquid in the etching tank for 10 to 15 minutes until the temperature of the liquid in the etching groove to be between 4 and 8 DEG C to obtain etching solution; and etching a polycrystalline silicon wafer in the etching solution for 280 to 320 seconds. According to the polycrystalline silicon wafer etching solution, the etching time is shortened; the polycrystalline etching effect is improved; the texture of the silicon wafer is uniform, fine and constant, the surface is clean, has good accordance without black lines; and the conversion efficiency and the quality of a solar cell are improved.
Owner:无锡尚品太阳能电力科技有限公司
Chemical corrosion transfer method based on GaN substrate CVD epitaxial growth graphene
ActiveCN103606514AShort corrosion timeLower corrosion temperaturePolycrystalline material growthSemiconductor/solid-state device manufacturingCvd grapheneCopper substrate
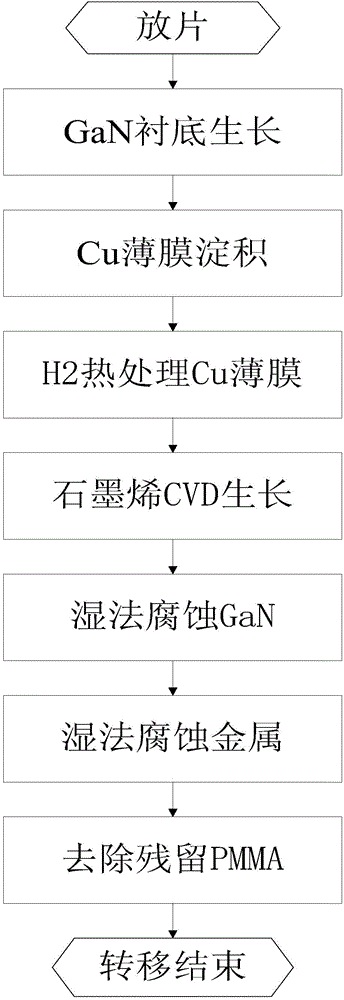
The invention discloses a chemical corrosion transfer method based on GaN substrate CVD epitaxial growth graphene. The problems that a copper substrate is wasted, and graphene transfer wastes time in the prior art are mainly solved. The chemical corrosion transfer method comprises the implementation steps that (1), a-face silicon carbide is arranged in an MOCVD reaction chamber, a gallium source and ammonia gas are filled into the MOCVD reaction chamber, and a-face GaN is grown; (2), a Cu thin film is deposited on the GaN through electron beam evaporation; (3), H2 is filled into the MOCVD reaction chamber, and thermal annealing is carried out on the Cu thin film; (4), H2 and CH4 are filled into the MOCVD reaction chamber, and the graphene is grown through chemical meteorology deposition; (5), the surface of the graphene is coated with photoresist in a spinning mode, the graphene is placed in potassium oxide solutions, a GaN middle layer is accelerated to be corroded with the help of lasers, and then the lower Cu thin film is corroded and removed; (6), the photoresist of the graphene with the thin film corroded and removed is upwards placed in an insulation substrate, the graphene is heated after being dried through air, then the graphene is cooled to indoor temperature and is placed in acetone to remove the photoresist on the upper surface of the graphene, and graphene transfer is achieved. The chemical corrosion transfer method has the advantages of being short in transfer time and saving the Cu substrate.
Owner:XIDIAN UNIV
Method for displaying M/A island structures inside pipeline steel
The invention discloses a method for displaying M / A island structures inside pipeline steel, and belongs to the technical field of metallographic phase structure displaying and quantifying. Specifically, A sample is grinded and polished into a mirror face to be used later; 1g sodium metabisulfite is added into 100ml distilled water inside a beaker; 3.65g picric acid and 0.65g sodium hydroxide are added into 100ml absolute ethyl alcohol; the two kinds of solutions are mixed according to the proportion of 3: 2 and are heated and stirred to obtain the improved staining reagent; 2-4% of nitric acid ethyl alcohol solution is used for slightly corroding the prepared sample, and the sample is placed into the staining reagent to be immersed for 120-240 seconds and then is washed clean through the ethyl alcohol and is blown to be dried; an M / A island presents bright white and is very different from the surrounding structures in the gray level. The method is easy to operate, the corrosion time is short, and the area content, the size, the shape and other characteristic parameters of the M / A island in the structures can be fast, accurately and quantificationally analyzed through metallographic phase software.
Owner:BC P INC CHINA NAT PETROLEUM CORP +1
Magnesium alloy metallographic corrosive agent
The invention relates to a magnesium alloy metallographic corrosive agent and belongs to the technical field of magnesium alloy metallographic tests. The magnesium alloy metallographic corrosive agent is composed of oxalic acid and the following components including, by size, 1-3 parts of nitric acid with the concentration being 68%, 2-4 parts of acetic acid with the mass percent concentration being 98% and 100 parts of ethyl alcohol; and 10-15 g of the oxalic acid is adopted for every 100 ml of the ethyl alcohol. The magnesium alloy metallographic corrosive agent can fast and effectively corrode magnesium alloy, and corrosive time can be accurately controlled.
Owner:HENAN UNIV OF SCI & TECH
Preparation method of magnesium alloy surface super-hydrophobic fluorine conversion coating
InactiveCN107740083AReduce corrosionLessen the tendency to corrodeMetallic material coating processesNano structuringSimulated body fluid
The invention discloses a preparation method of a magnesium alloy surface super-hydrophobic fluorine conversion coating. The super-hydrophobic fluorine conversion coating of a micro / nano structure isprepared on the surface of a magnesium alloy by the adoption of a liquid-phase growth and hydro-thermal treatment combined method. Through cleaning, polishing, preparation of a fluorine conversion layer and hydrophobization treatment, a porous hydrophobic coating of a micro / nano structure is formed on the film layer. The contact angle of the coating in simulated body fluid reaches 150 degrees, thecontact area between a magnesium alloy sample and the liquid can be reduced, the corrosion current density of the magnesium alloy can be lowered by 3 grades, and the impedance magnitude is increasedfrom 12000 omega.cm<2> to 1400000 Omega.cm<2> and is increased by 100 times. The preparation method of the hydrophobic coating of the nano-structure is simple, equipment is simple and easy to control,cost is low, and the controllability is good.
Owner:CHONGQING UNIV OF TECH
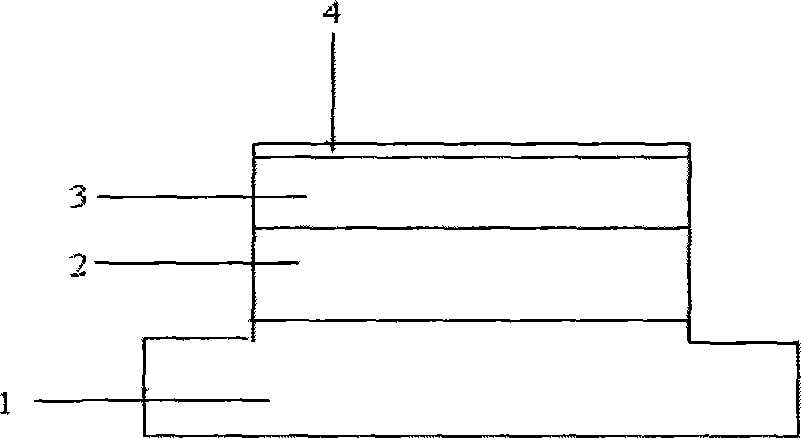
MEMS release auxiliary structure and preparation method thereof
InactiveCN105621342AReduce release lengthShort corrosion timeSemi-permeable membranesFixed microstructural devicesEngineeringAtmospheric pressure
The invention relates to an MEMS release auxiliary structure and a preparation method thereof. The structure comprises a bottom layer, and a second sacrificial layer and a second structure layer successively arranged on the bottom layer; the side of the bottom layer close to the second sacrificial layer is equipped with a plurality of grooves; the second sacrificial layer extends to the grooves and respectively forms hollow cavities in corresponding grooves; the air pressures in the hollow cavities are less than the air pressure in a corrosive environment; a plurality of corrosive agent inlets for communicating the outside world and the second sacrificial layer are arranged on the second structure layer; at least one corrosive agent inlet is a structure layer through hole or a release auxiliary hole; and the bottom layer is an underlay or is prepared by successively forming a sacrificial layer and a first structure layer on the underlay. According to the MEMS release auxiliary structure provided by the invention, the corrosive agent corrodes the second sacrificial layer through the hollow cavities towards the periphery; the release length is effectively reduced; and the corrosive time is reduced.
Owner:苏州工业园区纳米产业技术研究院有限公司
Polyphase structure high-grade pipeline steel original austenite grain boundary corrosion agent and application method
InactiveCN106191864AEliminate distractionsExtend corrosion aging timePreparing sample for investigationAustenite grainVolume concentration
The invention discloses a polyphase structure high-grade pipeline steel original austenite grain boundary corrosion agent and an application method. The corrosion agent consists of the following components: mew gull brand detergent, carbon tetrachloride, saturated picric acid solution, sodium chloride, hydrochloric acid solution with a volume concentration of 30%, and ethanol solution. The corrosion agent is convenient for preparation, needs no heating, can be prepared at normal temperature, is simple in operation, is short in corrosion time, is better in reproducibility, and is suitable for large-batch corrosion of polyphase structure pipeline steel.
Owner:SHANDONG IRON & STEEL CO LTD
Etching method of corrosion agent for displaying crystal boundary of quenched and tempered low-alloy chrome molybdenum steel austenite crystal grains
ActiveCN105865882AThere is no security riskNo safety hazardPreparing sample for investigationWater bathsAnhydrous ethanol
The invention provides an etching method of a corrosion agent for displaying the crystal boundary of quenched and tempered low-alloy chrome molybdenum steel austenite crystal grains. The method comprises the following steps: preparing the corrosion agent; putting absorbent cotton in a glass dish containing the corrosion agent to make the absorbent cotton adsorb the corrosion agent; cleaning a polished low-alloy chrome molybdenum steel sample, blowing the sample until the sample is dry, immersing the dry sample in the corrosion agent, arranging the immersed sample on the absorbent cotton in a manner that the polished surface of the sample is downward, immersing for 10-15min, taking out the sample, cleaning the sample with clear water, cleaning the sample with anhydrous ethanol, and blowing the cleaned sample until the sample is dry; and rating the grain size of the quenched and tempered low-alloy chrome molybdenum steel austenite crystal grains under a microscope. The method has the advantages of simple preparation of the corrosion agent, no need of water bath heating, realization of large-batch evaluation of the grain size grade in 10-15min, safe operation, and high convenience.
Owner:TIANJIN PIPE GROUP CORP
Corrosion liquid and corrosion method for original austenite grain boundary of medium carbon steel
PendingCN109001200AAffect the corrosion inhibition effectDosage controllablePreparing sample for investigationMaterial analysis by optical meansAustenite grainDistilled water
The invention discloses a corrosion liquid and a corrosion method for an original austenite grain boundary of medium carbon steel. The corrosion liquid comprises water, hydrochloric acid, picric acid,carbon tetrachloride, ethanol and LaFang shampoo in the ratio being (38-42 mL):(2-3 mL):(4-5 g):( 4-5 mL) (9-11 mL) (5-6 mL), wherein the concentration of hydrochloric acid is 36%-38% (by mass). Firstly, distilled water is put in a beaker, ethanol is added, hydrochloric acid, picric acid, carbon tetrachloride and LaFang shampoo are sequentially and slowly added randomly, and finally, the mixtureis stirred and mixed uniformly. The preparation method of the corrosion liquid is simple and the corrosion liquid can be stored for a long time.
Owner:UNIV OF JINAN
Metallographic corrosion method and metallographic corrosive agent for aluminum alloy
ActiveCN114318341APrevent smearingCorrosion method is simplePreparing sample for investigationHydrogen SulfateGrain boundary
The invention discloses an aluminum alloy metallographic corrosion method and a metallographic corrosive agent thereof. The metallographic corrosion method comprises the following steps that a, a 7055 aluminum alloy sample is subjected to pre-cleaning, hot inlaying, mechanical grinding and mechanical polishing treatment, washed with clear water and blow-dried, and then a metallographic sample is obtained; b, sequentially adding nitric acid, hydrochloric acid, sulfuric acid, hydrofluoric acid and deionized water into a container, and mixing to obtain a metallographic corrosive agent, wherein the metallographic corrosive agent comprises the following components in percentage by volume: 4-5% of nitric acid, 2-3% of hydrochloric acid, 1.5-2.5% of sulfuric acid, 2.5-3.5% of hydrofluoric acid and the balance of deionized water; and c, pouring the metallographic corrosive into a culture dish, placing the culture dish in a constant-temperature environment of 22-28 DEG C, immersing the metallographic sample into the metallographic corrosive, corroding for 25-35 seconds, taking out, washing, drying, and observing. The corrosion method is simple, the corrosion time is short, the effect of the metallographic corrosive agent is fully exerted, and the crystal boundary and crystal grains of the 7055 aluminum alloy are clearly displayed.
Owner:DONGFENG MOTOR GRP
Metallographic corrosion method for high-carbon martensitic stainless steel grain boundary
PendingCN111811912ADoes not affect grain size evaluationShort corrosion timePreparing sample for investigationTesting metal structuresAlcoholPolishing
The invention belongs to the field of metallographic analysis and detection of stainless steel, and particularly relates to a metallographic corrosion method for high-carbon martensitic stainless steel grain boundary. The problem that a metallographic corrosion method which aims at high-carbon martensitic stainless steel, is easy and convenient to operate and low in cost is lacked is solved. The invention provides the metallographic corrosion method of a high-carbon martensitic stainless steel grain boundary, wherein the method comprises the following steps: carrying out coarse grinding, finegrinding, polishing, cleaning and drying on a high-carbon martensitic stainless steel metallographic sample to obtain a bright scratch-free polished surface; and corroding the polished surface for 5-30 s at room temperature by adopting a metallographic corrosive agent, cleaning and drying. The metallographic corrosive agent is composed of 1 g to 4 g of picric acid, 5 ml of concentrated hydrochloric acid, 100 ml of ethyl alcohol and 2 ml to 5 ml of dishwashing liquid. The method aims at the high-carbon martensitic stainless steel, the grain boundary display effect is good, normal-temperature corrosion is adopted without pre-heating the sample, the corrosion time is short, operation is easy, and application and popularization are easy.
Owner:什邡新工金属材料有限公司
Metallographic corrosive agent for double-phase medium manganese steel and metallographic structure display method
ActiveCN112857950AGood corrosion effectEasy to knowPreparing sample for investigationMaterial analysis by optical meansCopper chlorideAustenite
The invention provides a metallographic corrosive agent for double-phase medium manganese steel and a metallographic structure display method. The metallographic corrosive is a mixed solution formed by copper chloride dihydrate, concentrated hydrochloric acid, absolute ethyl alcohol, sodium chloride, concentrated sulfuric acid and water. The metallographic structure display method comprises the following steps: providing a double-phase medium manganese steel sample; manually grinding and mechanically polishing the double-phase medium manganese steel sample; immersing the polished medium manganese steel sample in a metallographic corrosive at the temperature of 20 to 30 DEG C, slightly rotating and stirring, corroding for 10 to 20 s and then taking out; and cleaning the corroded medium manganese steel sample with detergent, alcohol and clear water sequentially and blow-drying, and carrying out observation through a metallographic microscope. The metallographic corrosive is simple in preparation method and convenient to use, the corrosion effect is good through the method, and the austenite ferritic structure effect of the two-phase medium manganese steel material can be clearly analyzed.
Owner:沧州赛腾特种材料科技有限公司
Preparation method of surface texture of polycrystalline silicon solar cell
InactiveCN101624700AImprove conversion efficiencyReduce surface reflectivitySemiconductor devicesAcid etchingHydrofluoric acid
The invention discloses a preparation method of the surface texture of a polycrystalline silicon solar cell. The surface texture of the polycrystalline silicon solar cell is prepared by an acid etching solution, the acid etching solution contains hydrofluoric acid, an oxidant and a modifier, wherein the concentration range of the hydrofluoric acid is from 13moles / litre to 17moles / litre; the oxidant is a potassium permanganate solution, and the concentration range of the oxidant is from 0.01moles / litre to 0.5moles / litre; the modifier is sodium nitrite, and the concentration range of the modifier is from 0.05moles / litre to 0.2moles / litre; the etching temperature is 10-30 DEG C, and the etching time is 10-45 minutes. The invention reduces the reflectivity of the polycrystalline silicon solar cell and has little environmental pollution.
Owner:CSI CELLS CO LTD +6
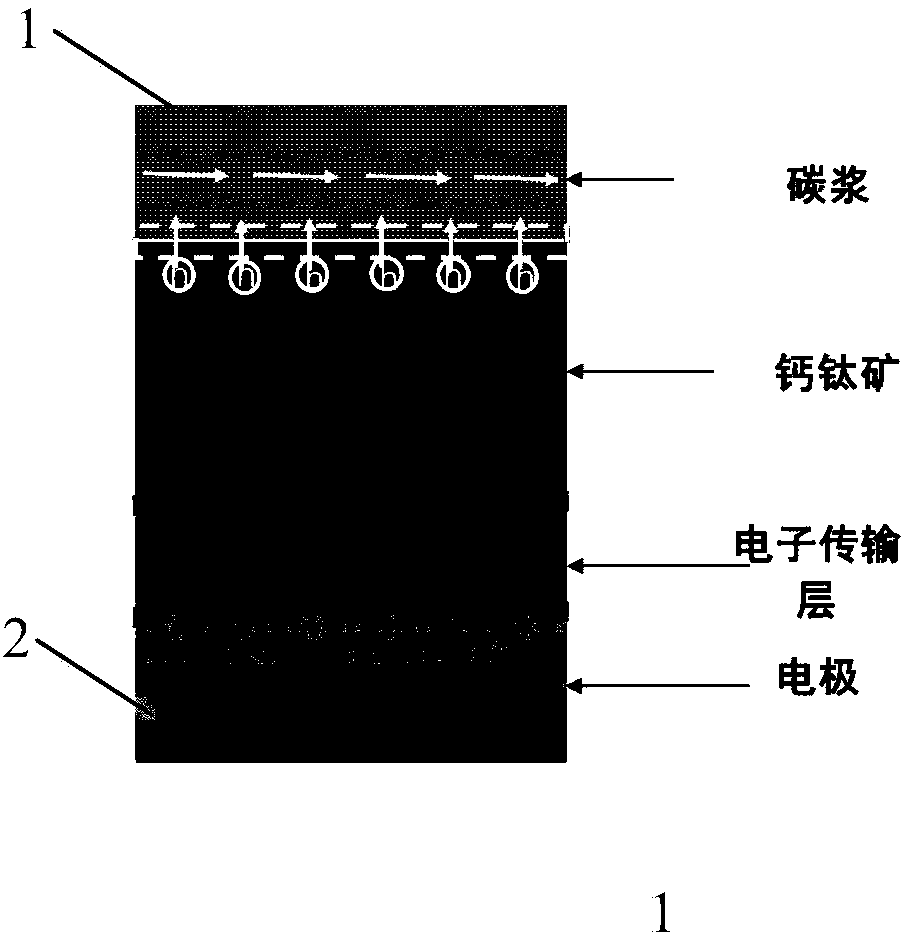
Carbon electrode perovskite solar battery and preparation method thereof
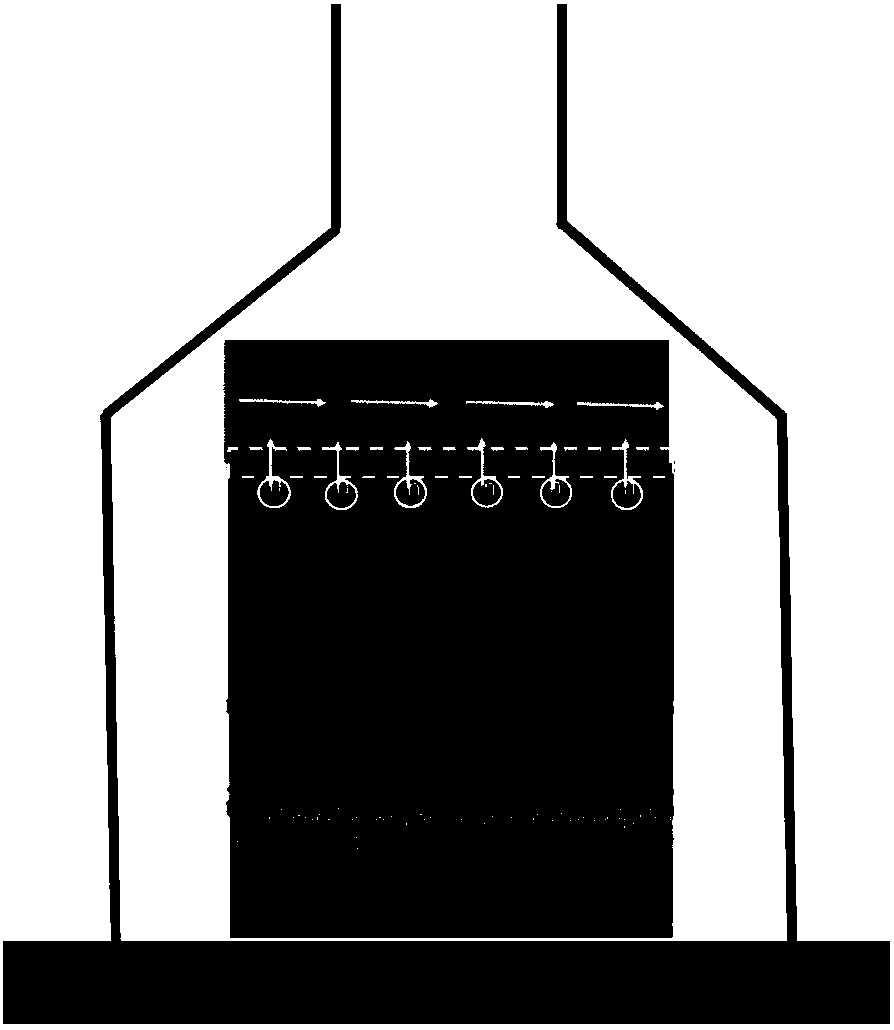
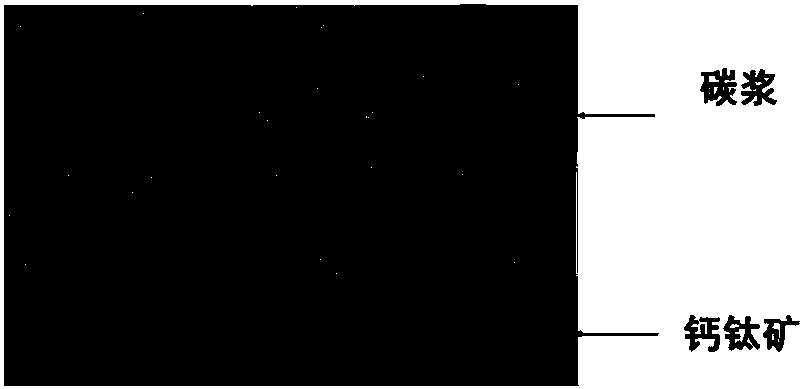
ActiveCN107910443AShorten drying timeLeave quicklySolid-state devicesSemiconductor/solid-state device manufacturingPerovskite solar cellGas phase
The invention discloses a carbon electrode perovskite solar battery and a preparation methodthereof. An electron transmission layer, a perovskite thin film, and(a hole transmission layer) are preparedon a transparent conducting substrate in sequence and then the transparent conducting substrate is coated with carbon pulp. Then air exhaust drying is performed on the carbon pulp and the stable andefficient carbon electrode perovskite solar battery is prepared. The corrosion time of perovskite corroded by a solvent in the carbon pulp is the drying time of the carbon pulp, so that the evaporation of the solvent in the carbon pulp is accelerated, the carbon pulp drying time is shortened, and corrosion on perovskite by the solvent in the carbon pulp can be reduced effectively. According to theinvention, aiming at different kinds of carbon pulps, a universal method is provided for solving the problem of corrosion on perovskite by the solvent in the carbon pulp, quick leaving of the solventvolatilized from the carbon pulp can be urged due to the differential pressure effect by utilizing an air exhaust method, so that the solvent can volatilize to a gas phase quickly further, the carbonpulp drying time is reduced substantially, and the corrosion on perovskite by the solvent is reduced. Therefore, a solid foundation is laid for preparing the stable and efficient carbon electrode solar battery.
Owner:咸阳瞪羚谷新材料科技有限公司
Method for producing air-gap structure by selective etching aluminum arsenide using dilute hydrochloric acid
InactiveCN101456531AModerate lateral corrosion rateUniform and stable lateral corrosion rateDecorative surface effectsPhotomechanical apparatusWater bathsMaterials science
The invention relates to a method for manufacturing an air gap structure by using dilute hydrochloric acid to selectively corrode an aluminum arsenide sacrificial layer, which comprises the following steps: a, growing a structure with the aluminum arsenide sacrificial layer on a gallium arsenide substrate by using a molecular beam epitaxial technique, and arranging a distributed Bragg reflector consisting of gallium arsenide and aluminum gallium arsenide on the aluminum arsenide sacrificial layer; b, photoetching a required graph on the surface of a wafer, non-selectively and vertically corroding a table top on the surface of the wafer, and exposing the side wall of the aluminum arsenide sacrificial layer; c, selectively corroding the aluminum arsenide sacrificial layer on the wafer by dilute hydrochloric acid in a thermostatic water bath; d, transferring the selectively corroded wafer to a beaker filled with methanol or acetone by a special polyfluorin porcelain perforated ladle, and cleaning the wafer; e, keeping the beaker filled with the methanol or the acetone standing in a constant temperature environment until the methanol or the acetone fully volatilizes, and taking out the wafer; and f, measuring the depth of corroding the aluminum arsenide at the side of the dilute hydrochloric acid, and estimating average rate of corroding the aluminum arsenide at the side of the dilute hydrochloric acid to obtain the required air gap structure.
Owner:INST OF SEMICONDUCTORS - CHINESE ACAD OF SCI
Test method of a high-purity aluminum alloy
PendingCN111751251AShort corrosion timeReduced Possibility of ContaminationPreparing sample for investigationParticle size analysisPhysical chemistryParticle counter
The invention discloses a test method of a high-purity aluminum alloy. The test method of the high-purity aluminum alloy comprises the following steps: 1) cutting a high-purity aluminum alloy sample into pieces, and carrying out impurity removal pretreatment; 2) weighing the pretreated high-purity aluminum alloy sample, adding a corrosive liquid for corrosion, standing, and adding water for dilution to form a mixed sample solution; 3) weighing the mixed sample solution, adding water to dilute the mixed sample solution, detecting the granularity of the sample solution through a liquid particlecounter for at least three times, and taking an average value S1; and 4) adding water into hydrochloric acid to form a hydrochloric acid solution with the same proportion as the mixed sample solutionin the step (3), taking the hydrochloric acid solution, adding water to dilute, detecting through a liquid particle counter, detecting for at least three times, taking an average value S2, and calculating the granularity of the sample. According to the test method of the high-purity aluminum alloy, the corrosion time of a high-purity aluminum alloy sample is shortened, the detection efficiency isimproved, the possibility that sample liquid is polluted is reduced, and data are more stable.
Owner:KONFOONG MATERIALS INTERNATIONAL CO LTD
Method for preparing two-dimensional nanosheet crystal Ti3C2 alkene
InactiveCN106744961AWell mixedReduce the binding forceTitanium carbideHydrogen fluorideMechanical crushing
The invention discloses a method for preparing two-dimensional nanosheet crystal Ti3C2 alkene. The method specifically comprises the following steps: performing mechanical crushing and grinding on a high-purity Ti3AlC2 block, and screening by using a sieve of 200 meshes so as to obtain Ti3AlC2 raw material powder of which the particle size is smaller than 75[mu] m; mixing the Ti3AlC2 raw material powder and 10-40wt% HF (Hydrogen Fluoride) acid, sealing, stirring, heating to 25-60 DEG C, and further stirring to react for 0.5-10 hours at 25-60 DEG C so as to obtain a liquid mixture; performing multiple times of centrifugal treatment on the liquid mixture till the pH value of supernate obtained after centrifugal treatment is 5-7 so as to obtain a crude product; performing vacuum drying on the crude product, thereby obtaining the two-dimensional nanosheet crystal Ti3C2 alkene. Compared with other documents and patents, the method aims to prepare raw material powder with low-concentration acid corrosion and a relatively wide particle size range from the Ti3AlC2 raw material powder of which the particle size is smaller than 75[mu] m by using a low-concentration acid solution in modes of continuous stirring and heating heat preservation, not only is the particle size range of the raw material powder increased, but also the corrosion time is greatly shortened, and great significances for promoting industrial production and actual application of MXene can be achieved.
Owner:BEIJING JIAOTONG UNIV
Ultra-low carbon steel metallographic corrosive agent and display method of metallographic structure
PendingCN113984473AShort corrosion timeEtching steps are simplePreparing sample for investigationMaterial analysis by optical meansAcetic acidCarbon steel
The invention discloses a preparation method of an ultra-low carbon steel metallographic corrosive agent and a display method of a metallographic structure, and relates to the technical field of metallographic preparation. The corrosive agent is composed of 90% by volume of absolute ethyl alcohol, 5% by volume of nitric acid and the balance of glacial acetic acid, and can be obtained after the substances are evenly mixed. The display method of the metallographic structure is a one-step corrosion method and comprises the following steps: firstly, blow-drying water stains on the surface of an ultra-low carbon steel sample subjected to fine polishing, then directly eroding the ultra-low carbon steel sample for 50 seconds by using a corrosive agent at normal temperature, rinsing the surface of the sample by using clear water, blow-drying the sample, and observing the metallographic structure. The metallographic corrosive agent provided by the invention is short in corrosion time, simple in corrosion step and convenient to operate, can enable the grain boundary of grains in ultra-low carbon steel to be clear, and is beneficial to the measurement of the grain size.
Owner:唐山瑞丰钢铁(集团)有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com