A kind of method for preparing gemcitabine hydrochloride
A technology of gemcitabine hydrochloride and its synthesis method, which is applied in the field of drug synthesis, and achieves the effects of high purity, simple and easy post-processing operation, high purity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Add 500g of GE-0 into a 5L three-necked flask, then add 2.5L of ethyl acetate, and gradually add 500g of tri-tert-butoxy lithium aluminum hydride in batches under mechanical stirring at 0-10°C (the reaction temperature should not exceed 10°C when adding) , After about 1-2 h, the addition was completed, and then the reaction was continued at 0-10°C for 4 hours. TLC monitors the end point of the reaction. After the reaction is completed, add 200 ml of concentrated hydrochloric acid dropwise under mechanical stirring at 0-10°C (the temperature of the reaction solution should not exceed 10°C). After the addition is complete, filter with suction and wash the filter cake with 1.5L*3 , the filtrates were combined, washed twice with saturated brine 2L*2, the ethyl acetate layer was separated, and the organic layer was concentrated to dryness to obtain 475 g of light yellow oil with a yield of 94.5%.
Embodiment 2
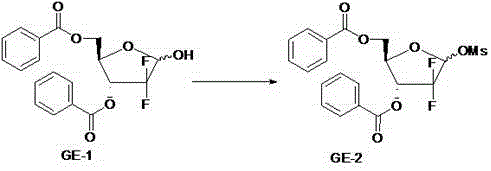
[0037] Add 475g GE-1 into a 5L three-necked flask, then add it into 2.5L dichloromethane, cool down to 5-10°C with a low-temperature cooling circulation pump, add 261ml triethylamine under mechanical stirring, drop by drop under mechanical stirring at 0-10°C 117ml of methanesulfonyl chloride was added, and the addition was completed in about 1-2 hours, and then the reaction was continued at 5-10°C for 4 hours. After the reaction was completed, wash with 2 L of 1 mol / L hydrochloric acid aqueous solution, 2 L of 5% sodium bicarbonate aqueous solution, and 2 L of Hao water, respectively, and concentrate the organic layer to dryness to obtain 566 g of light brown oil (crystals precipitated after standing for 1-2 days). The yield was 98.8%.
Embodiment 3
[0039] Add 275g of cytosine and 0.5g of ammonium sulfate into the reaction flask, then add 1.65L of hexamethyldisilazane, heat to reflux (120-130°C), continue to stir for 2 hours after the reaction liquid is clarified, cool to room temperature, and filter with suction , and the filter cake was vacuum-dried to obtain 605 g of N, O-bis(trimethylsilane)cytosine with a yield of 95%.
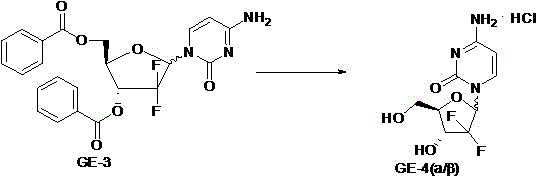
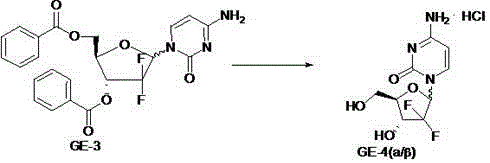
[0040] Add 565g of N,O-bis(trimethylsilane)cytosine into a 5L three-necked flask, then add 1.15L of anisole, stir well, then add 1.15L of trimethylsilyl trifluoromethanesulfonate, heat After the reaction solution is completely dissolved at about 125°C, dissolve 565g of GE-2 in 1.15L of anisole and add it dropwise to the reaction solution for about 1-2 hours, then continue the reaction under reflux for 5 Hour. After the reaction is complete, add 2.3L ethyl acetate, extract 2 times with 100L purified water, concentrate the organic layer to dryness to obtain a wine-red viscous substance, then add 1.4L ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com