ph-sensitive polymeric micelle composition resisting tumor drug resistance
An anti-tumor drug and polymer glue technology, which is applied in the field of anti-tumor drug-resistant pH-sensitive polymer micelle composition, can solve problems such as drug resistance and insufficient drug accumulation, and achieve the effect of achieving effectiveness and safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
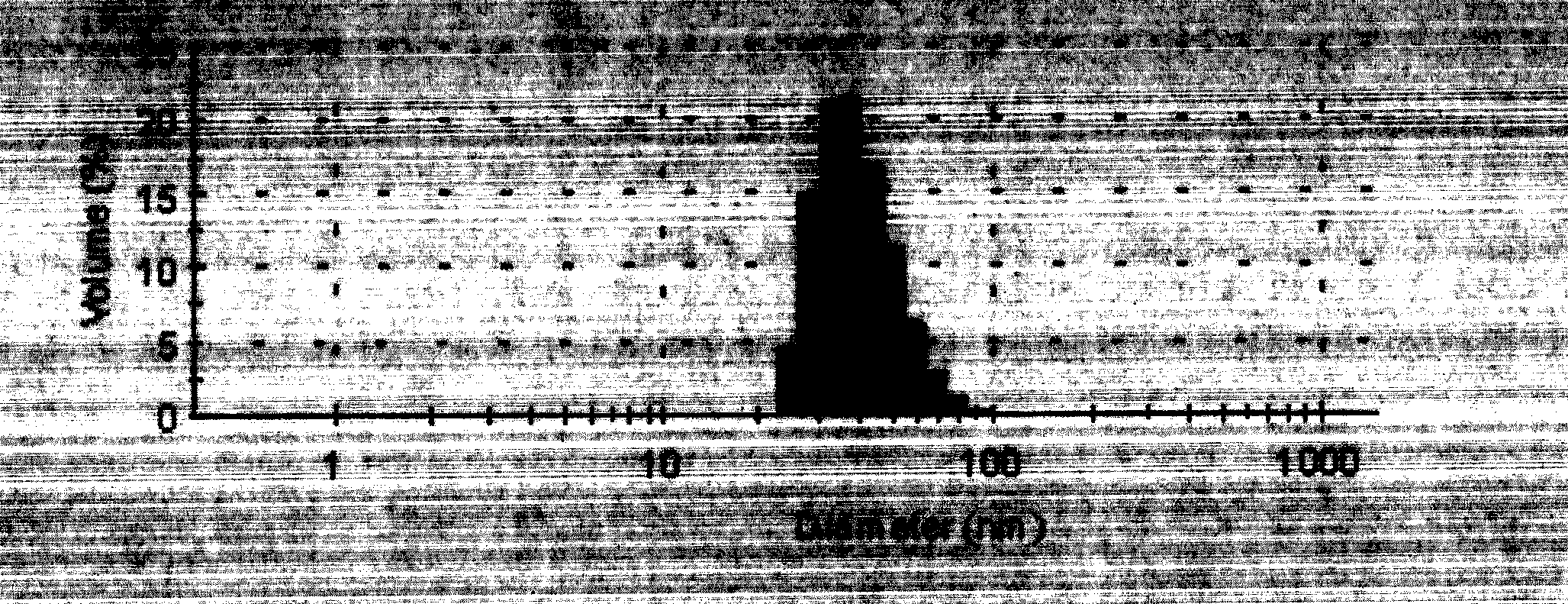
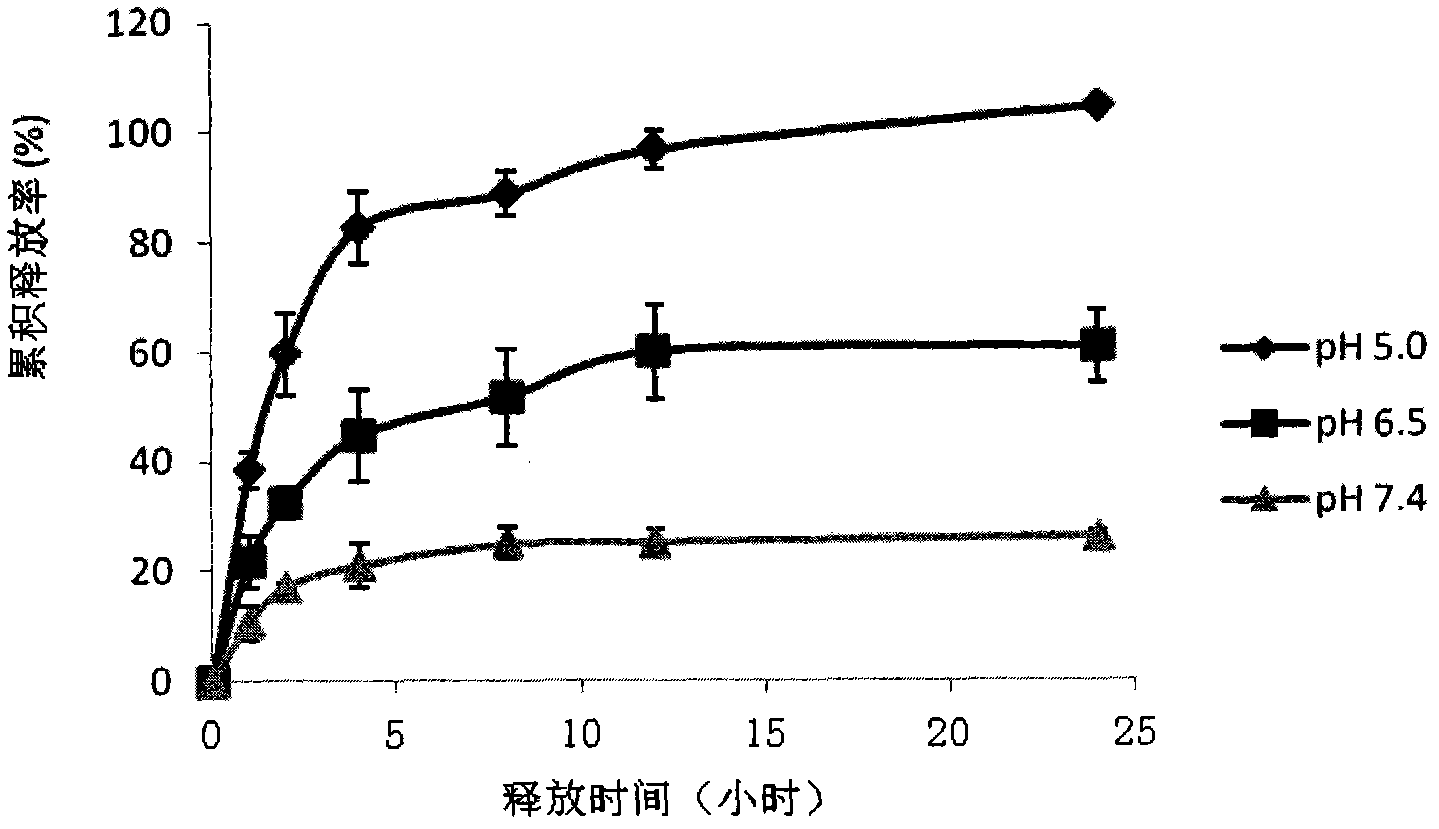
Image
Examples
Embodiment 1
[0028] Example 1 Synthesis of PEOz2000-VES
[0029] Methyl p-toluenesulfonate (150 mg) and 2-ethyl-2-oxazoline (5 g) were dissolved in acetonitrile (80 mL), and heated and stirred at reflux for 12 h under a nitrogen atmosphere. After cooling to room temperature, use NH 3 As a terminator, cold ether is precipitated and refined and dried in vacuum to prepare PEOz (PEOz-NH) with amino end groups and a molecular weight of 2000 2 ). Take appropriate amount of VES, EDC and NHS, and react in dichloromethane for 2 hours to obtain the active ester of VES (VES-NHS). Then add PEOz-NH in an appropriate molar ratio 2 , Using TEA as a catalyst, after 4 hours of reaction, precipitation and purification with cold ether, and vacuum drying to obtain the PEOz2000-VES of the present invention.
Embodiment 2
[0030] Example 2 Synthesis of poly(2-ethyl-2-oxazoline)-polylactic acid-poly(2-ethyl-2-oxazoline) (PEOz5000-PLA3000-PEOz5000)
[0031] In a three-necked flask equipped with a stirrer, add 2-ethyl-2-oxazoline (10g, 150mmol), acetonitrile (40mL), and methyl p-toluenesulfonate (0.47g) at an oil bath temperature of 80°C. Under nitrogen atmosphere, the reaction was stirred for 30h. After cooling, the methanol solution of KOH was added, and the reaction was continued for 4 hours. The solvent was removed, the residue was dissolved in THF, passed through a silica gel column, and the effluent was poured into excess cold ether for precipitation, suction filtration, and vacuum drying for 12 hours. The obtained PEOz-OH powder (4g) was dissolved in chlorobenzene (80mL), D,L-lactide (6.3g) and stannous octoate (0.63g) were added under nitrogen atmosphere, and reacted at 140°C for 24h. The reaction solution was poured into excess ether for precipitation, filtered, and vacuum dried at room tem...
Embodiment 3
[0032] Example 3 Synthesis of polylactic acid-poly(2-ethyl-2-oxazoline)-polylactic acid (PLA2000-PEOz8000-PLA2000)
[0033] 2-Ethyl-2-oxazoline (9.9 g) and 1,4-dibromo-2-butene (420 mg) were dissolved in acetone (40 mL), and stirred and refluxed at 100° C. for 20 h under a nitrogen atmosphere. After cooling to room temperature, 0.1 mol / L KOH methanol solution (40 mL) was added to the reaction flask, and the reaction was continued for 4 hours. After passing through a silica gel column, the effluent is poured into excess cold ether for precipitation, suction filtration, and vacuum drying for 24 hours. The obtained HO-PEOz-OH powder (2g) was dissolved in chlorobenzene (20mL), D, L-lactide (0.58g) and stannous octoate (30mg) were added under nitrogen atmosphere, and reacted at 140℃ for 24h . The reaction solution was poured into excess ether for precipitation, filtered, and vacuum dried at room temperature for 12 hours to obtain the triblock copolymer PLA2000-PEOz8000-PLA2000 of th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com