Normal-pressure CO2 catalytic conversion cyclic carbonate synthesis method
A cyclic carbonate, catalytic conversion technology, applied in the field of catalytic chemistry, can solve the problems of catalyst recycling pollution, etc., achieve the effect of low price, reduce environmental pollution, and easy recycling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
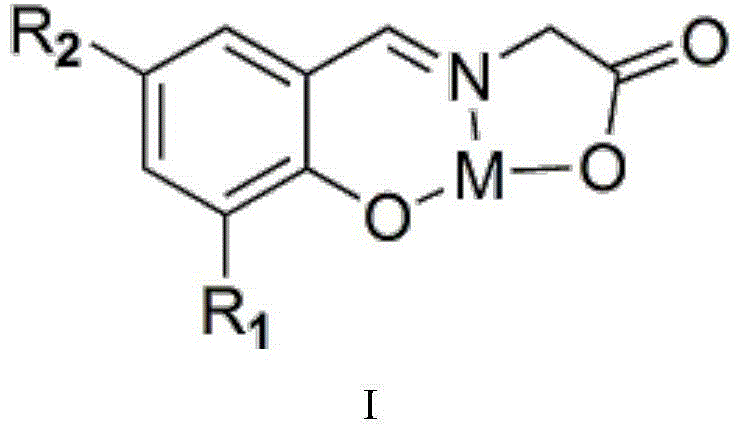
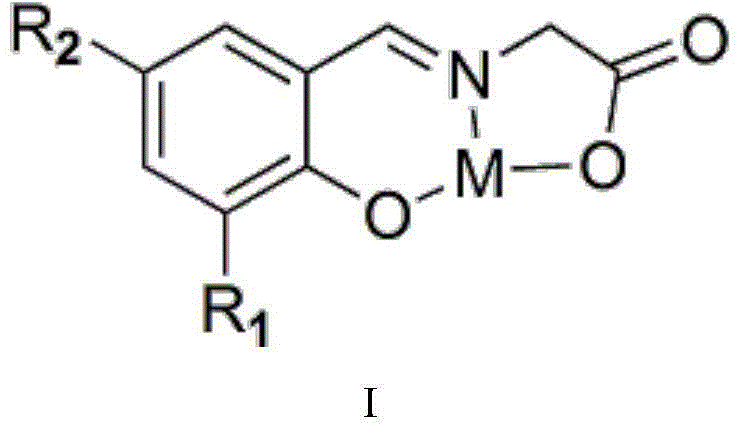
[0033] (1) The synthesis steps of catalyst (3-methylsalicylaldehyde glycine) combined with zinc are as follows: take glycine and sodium hydroxide solid 10mmol respectively and mix them in a 500mL round bottom flask, add 200mL absolute ethanol, and heat to 80°C , condensed and refluxed for 1 hour under magnetic stirring, and the solids in the solution disappeared completely to obtain an anhydrous ethanol solution of sodium glycinate; 10 mmol of 3-methyl salicylaldehyde was added thereto, heated to 80°C, and condensed and refluxed for 1 hour under magnetic stirring , a bright yellow solution was generated, at which time the solid disappeared completely, and an ethanol solution of the ligand (3-methylsalicylglycine) was obtained. After that, 10 mmol Zn(NO 3 ) 2 heated to 80°C, condensed and refluxed for 1 h under magnetic stirring, no precipitation occurred, cooled to room temperature, filtered to obtain a solid, alternately washed with ice water and ice ethanol until the washin...
Embodiment 2
[0036] (1) The synthetic steps of catalyst (3-fluorosalicylaldehyde glycine) chromium synthesis are: get glycine and sodium hydroxide solid 10mmol respectively and mix in a 500mL round bottom flask, add 200mL absolute ethanol, heat to 80°C, Condensation and reflux reaction under magnetic stirring for 1h, the solids in the solution completely disappeared, and an anhydrous ethanol solution of sodium glycinate was obtained; 10mmol of 3-fluorosalicylaldehyde was added to it, heated to 80°C, and condensing and reflux reaction was performed under magnetic stirring for 1h to generate A bright yellow solution, at which time the solid completely disappeared, and an ethanol solution of the ligand (3-fluorosalicylglycine) was obtained. After that, 10 mmol Cr(NO 3 ) 3heated to 80°C, condensed and refluxed for 1 h under magnetic stirring, no precipitation occurred, cooled to room temperature, filtered to obtain a solid, washed alternately with ice water and ice ethanol until the washing l...
Embodiment 3
[0039] (1) The synthesis steps of catalyst (5-hydroxysalicylaldehyde glycine) manganese compound are: get glycine and sodium hydroxide solid 10mmol respectively and mix in 500mL round bottom flask, add 200mL absolute ethanol, heat to 80°C, Condensation and reflux reaction under magnetic stirring for 1h, the solid in the solution disappeared completely, and an anhydrous ethanol solution of sodium glycinate was obtained; 10mmol of 5-hydroxy salicylaldehyde was added to it, heated to 80°C, and condensing and reflux reaction was performed for 1h under magnetic stirring to generate A bright yellow solution, at which time the solid completely disappeared, and an ethanol solution of the ligand (5-hydroxysalicylglycine) was obtained. After that, 10 mmol Mn(NO 3 ) 2 heated to 80°C, condensed and refluxed for 1 h under magnetic stirring, no precipitation occurred, cooled to room temperature, filtered to obtain a solid, alternately washed with ice water and ice ethanol until the washing...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com