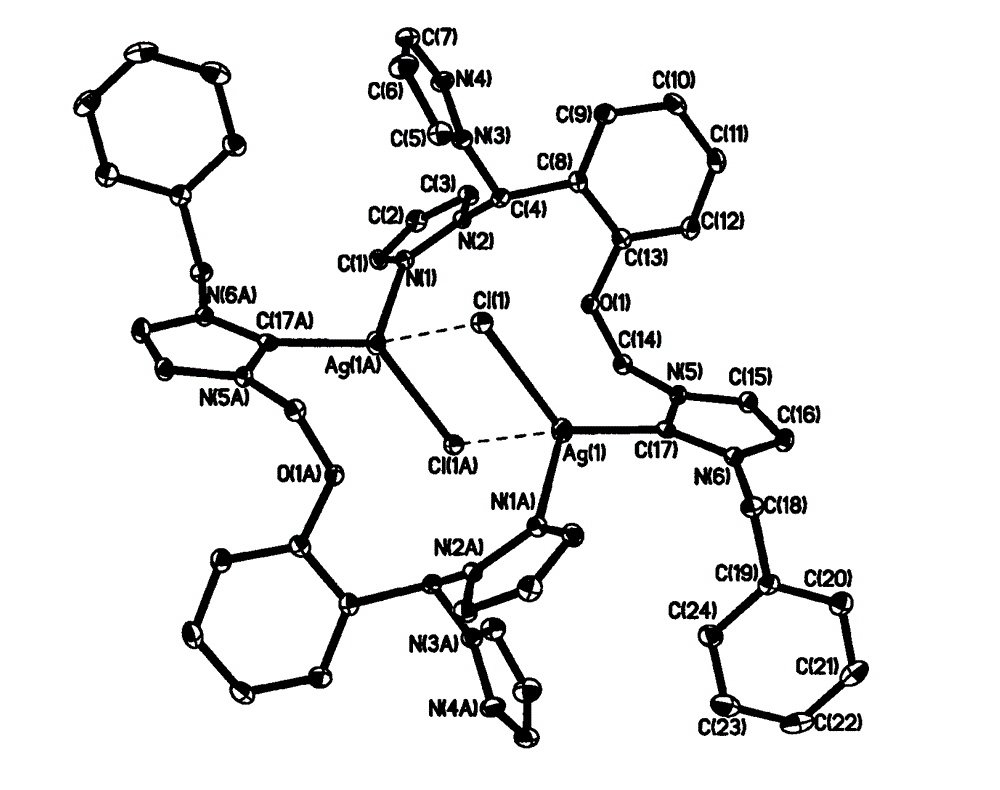
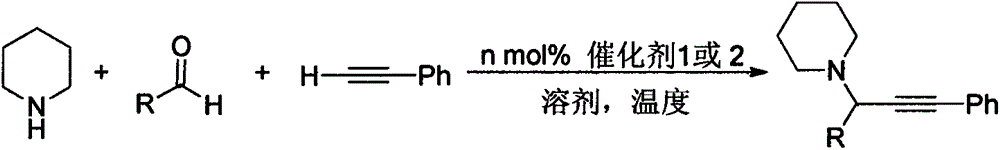Nitrogen heterocyclic carbene silver complex based on bispyrazole methyl phenoxy methylimidazole and preparation method and application thereof
A technology of bispyrazole methylphenoxymethylimidazole and methylphenoxymethyl is applied in the field of preparation of nitrogen heterocyclic carbene silver complexes, can solve rare problems and the like, and achieve strong fluorescence emission and efficient catalytic activity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Synthesis of 1-[2-bis(pyrazol-1-yl)methylphenoxymethyl]imidazole
[0015] In a 100mL round bottom flask, add 20mL of ethanol, 2.4g (10mmol) of 2-hydroxyphenylbis(pyrazol-1-yl)methane, 1.53g (10mmol) of 1-chloromethylimidazole hydrochloride and hydroxide Potassium 1.12g (20mmol), then heated and stirred under reflux for 24 hours. Cool to room temperature, filter, and wash the solid with ethanol (3×10 mL), which is the inorganic salt potassium chloride. The filtrate was removed from the solvent, and 20 mL of water was added, extracted with dichloromethane (3×30 mL), the organic phases were combined, dried over anhydrous sodium sulfate, and filtered. The solvent was removed to obtain 1.79 g of a light yellow solid product with a yield of 56%. 1 H NMR (400MHz, CDCl 3 ): δ5.97(s, 2H), 6.32(t, 2H), 6.89(s, 1H), 7.73(s, 1H), 7.94(s, 1H), 6.87(d, 1H), 7.04(t, 1H), 7.32(d, 1H), 7.39-7.45(m, 1H), 7.12(s, 1H), 7.56(d, 2H), 7.66(d, 2H).
[0016] Synthesis of Chloro-N-[2-bis(py...
Embodiment 2
[0022] Synthesis of N-[2-bis(pyrazol-1-yl)methyl]phenoxymethyl-N'-benzyl imidazolium tetrafluoroborate
[0023] In a 100mL round bottom flask, 0.45g (1.0mmol) of chloro N-[2-bis(pyrazol-1-yl)methyl]phenoxymethyl-N'-benzyl imidazolium salt (L1) was dissolved in 10 mL of water, and then 0.14 g (1.3 mmol) of ammonium tetrafluoroborate in 10 mL of aqueous solution was added dropwise to the above reaction mixture, and stirring was continued at room temperature for 3 hours after the addition was complete. It was extracted with dichloromethane (3×10 mL), and the organic phases were combined and dried over anhydrous sodium sulfate. After filtration, the solvent was removed from the filtrate under reduced pressure to obtain 0.45 g of a white hygroscopic solid product with a yield of 90%. 1 H NMR (400MHz, CDCl 3 ): δ5.24(s, 2H), 6.00(s, 2H), 6.23(t, 2H), 6.84(d, 1H), 7.15(d, 1H), 6.70-7.07(m, 3H), 7.31- 7.37 (m, 6H), 7.96 (s, 1H), 7.42 (d, 2H), 7.51 (d, 2H), 8.97 (s, 1H).
[0024] S...
Embodiment 3
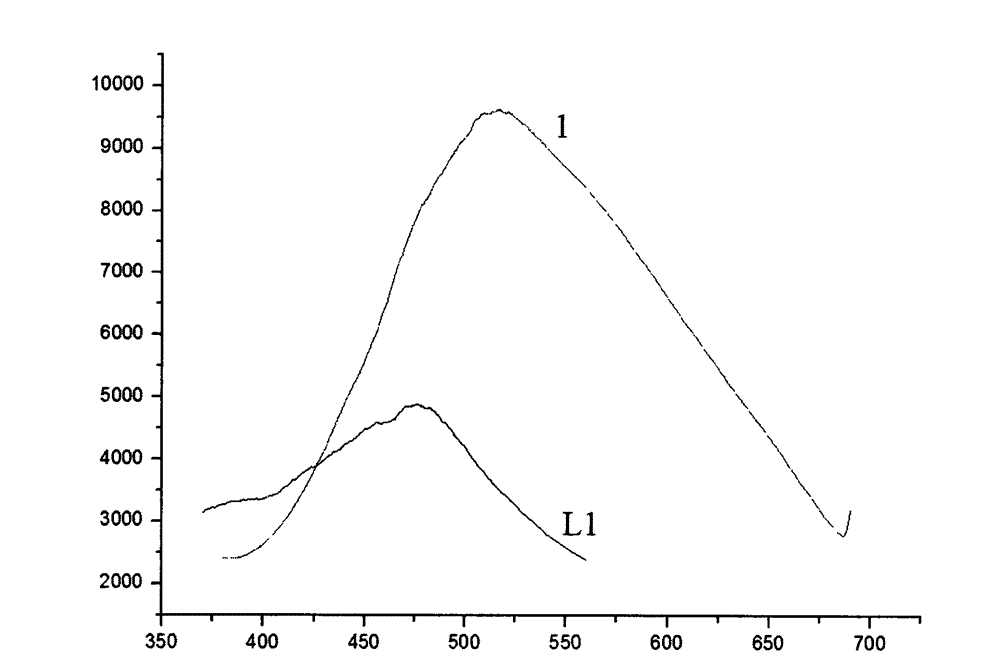
[0027] The ligand (L1) prepared in Example 1 and the corresponding nitrogen-heterocyclic carbene silver complex (1) were measured for solid fluorescence at room temperature, and the fluorescence emission spectrum intensity of the complex (1) was stronger than that of the corresponding ligand. Significant changes in fluorescence. The excitation wavelength of the ligand (L1) is 295nm and the excitation wavelength of the corresponding complex (1) is 360nm. The solid-state fluorescence diagram is shown in the appendix of the manual. figure 2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com