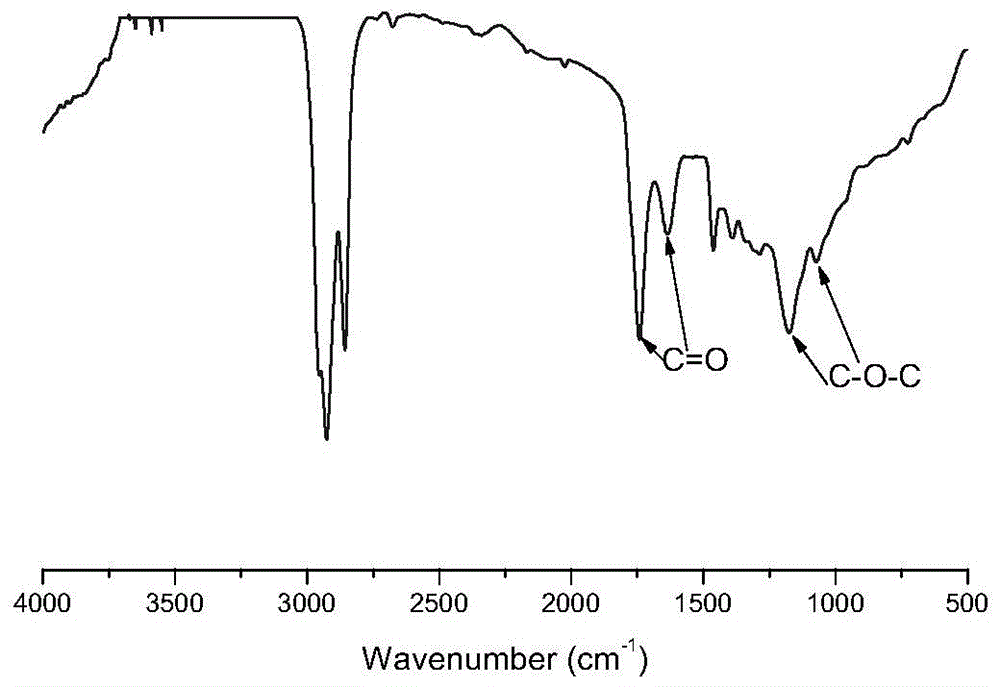
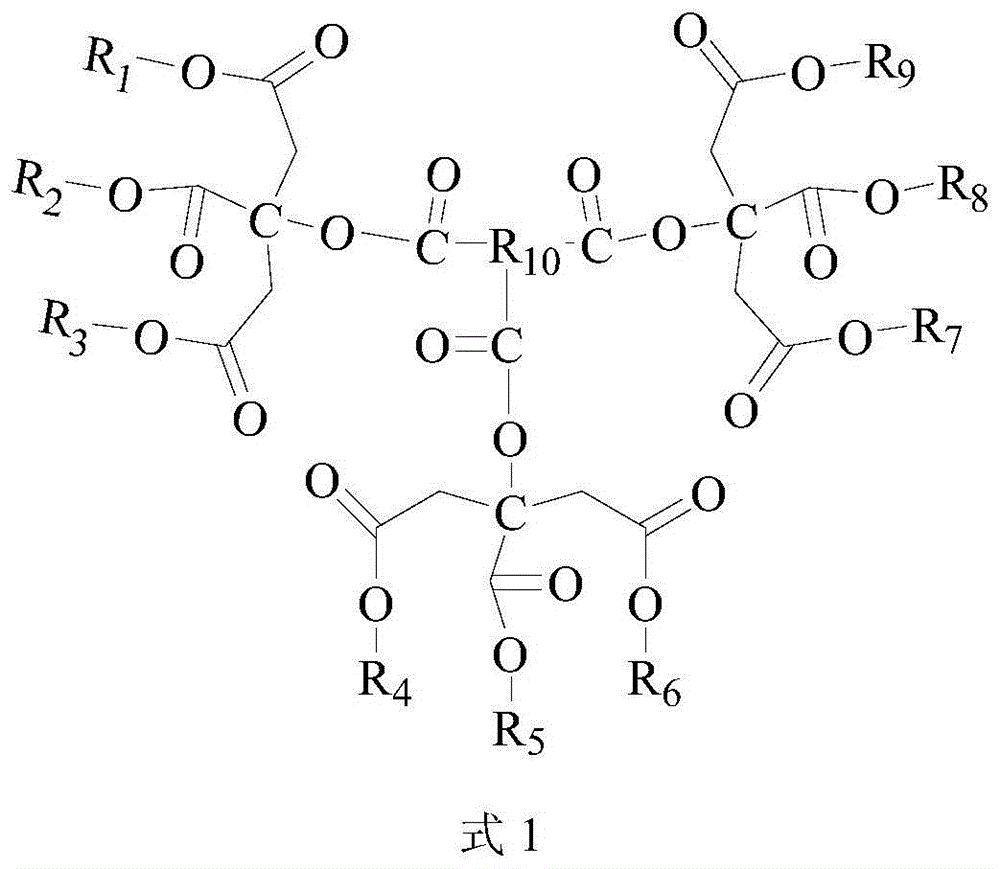
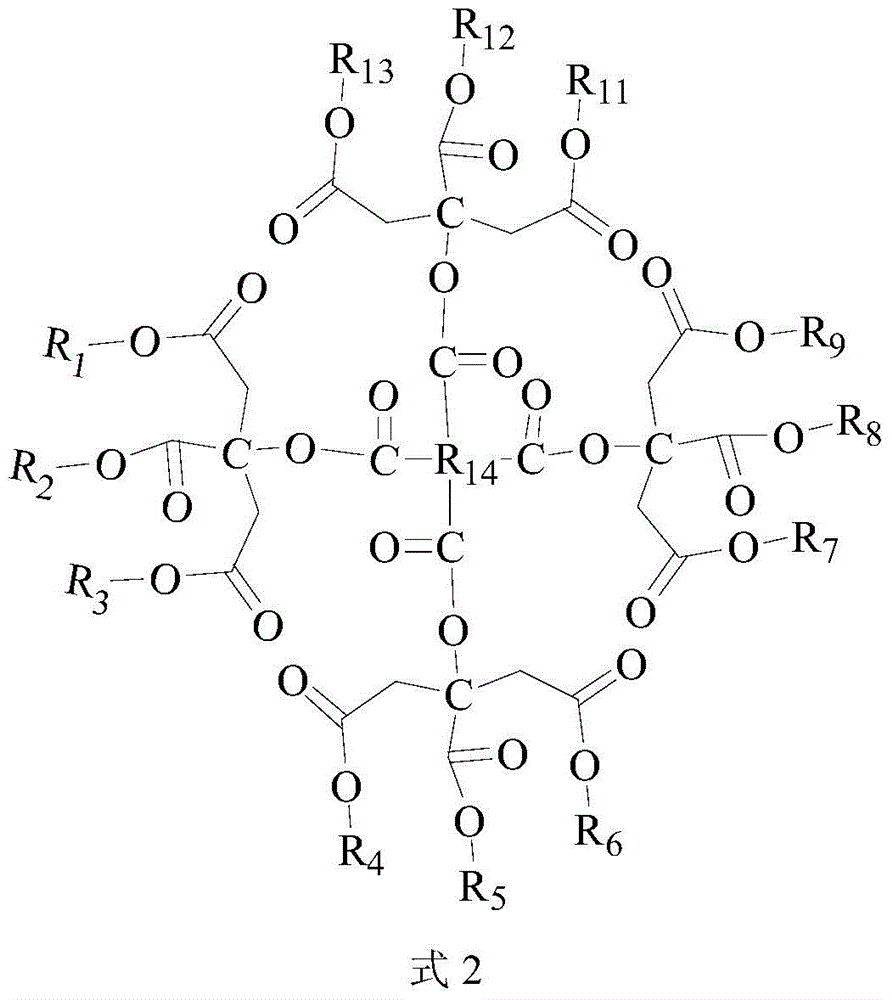
A kind of polycitrate triester and preparation method thereof
A technology of citric acid three and citric acid, applied in the field of plastic rubber plasticizers, can solve the problems of poor solvent extraction resistance and migration resistance, poor solvent extraction resistance and migration resistance, and failure to overcome thermal stability and the like, Achieve good migration resistance, meet industrial production requirements, and increase the effect of relative molecular weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The preparation of tributyl trimesic acid tricitrate comprises the following steps:
[0040] 1) Preparation of tributyl citrate: add 100mol citric acid, 380mol butanols, p-benzenemethanesulfonic acid (mass fraction is 2% of the total mass of citric acid and alcohol) and 40L water-carrying agent cyclohexane in the reactor , heated to 120°C under stirring, refluxed for 4 hours, recovered excess alcohol by distillation under reduced pressure to obtain crude tributyl citrate, then washed the crude tributyl citrate with 2% sodium bicarbonate solution, removed the catalyst, and recovered the organic phase to obtain Tributyl Citrate.
[0041] 2) Preparation of trimesoyl chloride: 70 mol of trimesic acid was added to the reaction kettle, 210 mol of phosphorus trichloride was added dropwise, the temperature was raised to 60° C. with stirring, cooled to room temperature, and trimesoyl chloride was obtained by standing and stratifying.
[0042] 3) Preparation of tributyl trimesic...
Embodiment 2
[0044] The preparation of tributylmalonate tricitrate comprises the following steps:
[0045] 1) Preparation of tributyl citrate: add 100mol citric acid, 400mol butanols, p-benzenemethanesulfonic acid (mass fraction is 2% of the total mass of citric acid and alcohol) and 40L water-carrying agent cyclohexane in the reactor , heated to 140°C under stirring, refluxed for 4 hours, and recovered excess alcohol by distillation under reduced pressure to obtain crude tributyl citrate, then washed the crude tributyl citrate with 2% sodium bicarbonate solution, removed the catalyst, and recovered the organic phase to obtain Tributyl Citrate.
[0046] 2) Preparation of glycerinyl chloride: add 70 mol of glyceric acid into the reaction kettle, dropwise add 210 mol of phosphorus trichloride, stir and heat up to 60° C., cool to room temperature, stand and separate to obtain glycerinyl chloride.
[0047] 3) Preparation of tributyl malonate tricitrate: add 100mol tributyl citrate and an appr...
Embodiment 3
[0049] The preparation of trioctyl trimesic acid tricitrate comprises the following steps:
[0050] 1) Preparation of trioctyl citrate: add 100mol citric acid, 380mol octanol, p-benzenemethanesulfonic acid (mass fraction is 2% of the total mass of citric acid and alcohol) and 40L water-carrying agent cyclohexane in the reactor , heated to 150°C under stirring, refluxed for 4 hours, recovered excess alcohol by distillation under reduced pressure to obtain crude trioctyl citrate, then washed the crude trioctyl citrate with 2% sodium bicarbonate solution, removed the catalyst, and recovered the organic phase to obtain Trioctyl Citrate.
[0051] 2) Preparation of trimesoyl chloride: 70 mol of trimesic acid was added to the reaction kettle, 200 mol of phosphorus trichloride was added dropwise, the temperature was raised to 60° C. with stirring, cooled to room temperature, and trimesoyl chloride was obtained by standing and stratifying.
[0052] 3) Preparation of trioctyl trimesic ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com