Production method of sargassum fusiforme lozenge for oral administration
A hijiki and slice processing technology, which is applied in the field of food deep processing, can solve the problems of hidden safety hazards and residual impurities, and achieve the effects of low bacterial content, improved purity, and convenient consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The processing method of hijiki oral buccal tablets comprises the following steps: (1) soaking and cleaning, placing the hijiki material in clean water, washing away the salt and sediment on the surface of the material, the weight ratio of clean water and hijiki is 1:3, soaking Time 2h; (2) Cooking, take out the soaked material and drain it and put it in a steamer for cooking, the cooking temperature is 100°C, and the cooking time is 2h; (3) Vacuum drying, put it in an airtight container to heat and dry and pump Vacuum, the pressure in the container remains below 700Pa (preferably 600Pa), the drying time is 40min, and the moisture content of the material is measured at 13% after completion; (4) Screening, the material is screened through a vibrating sieve, the number of vibrating sieves is 30 orders, and the buds and ear Separating from the stem and removing the stem, and keeping the bud ear; (5) crushing, crushing the screened material through a pulverizer, so that the ...
Embodiment 2-6
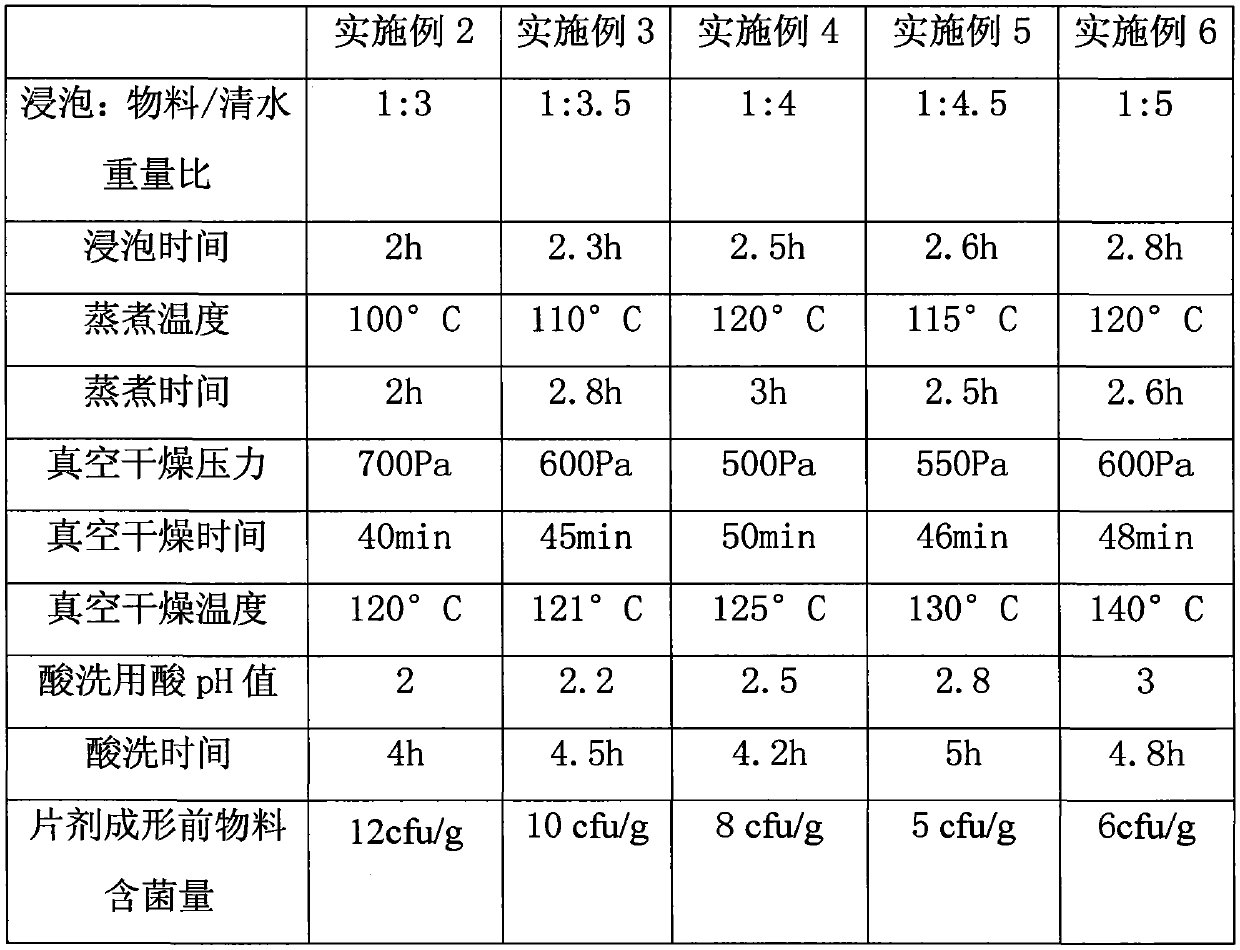
[0031] Examples 2-6 Refer to Table 1 for the corresponding parameters of each step.
[0032]
[0033] Table 1
[0034] The national standard for food bacteria content is required to be below 100cfu / g, and the Hijiki materials prepared by this process are all far below the bacteria content allowed by the national standard. And the active ingredients of the material before tablet forming are basically consistent with the aforementioned research results.
[0035] The auxiliary materials selected in Examples 2-6 also include the following parts by weight: 0.5% to 0.6% of citric acid, 5% to 15% of soluble starch, 7% to 8% of dextrin, 0.6% to 0.8% of sodium carboxymethylcellulose %, added as appropriate according to the product grade. The excipients in this range can not only retain the unique flavor, but also improve the taste, improve the physical properties of the final oral lozenge, and are suitable for long-term oral administration.
Embodiment 7-11
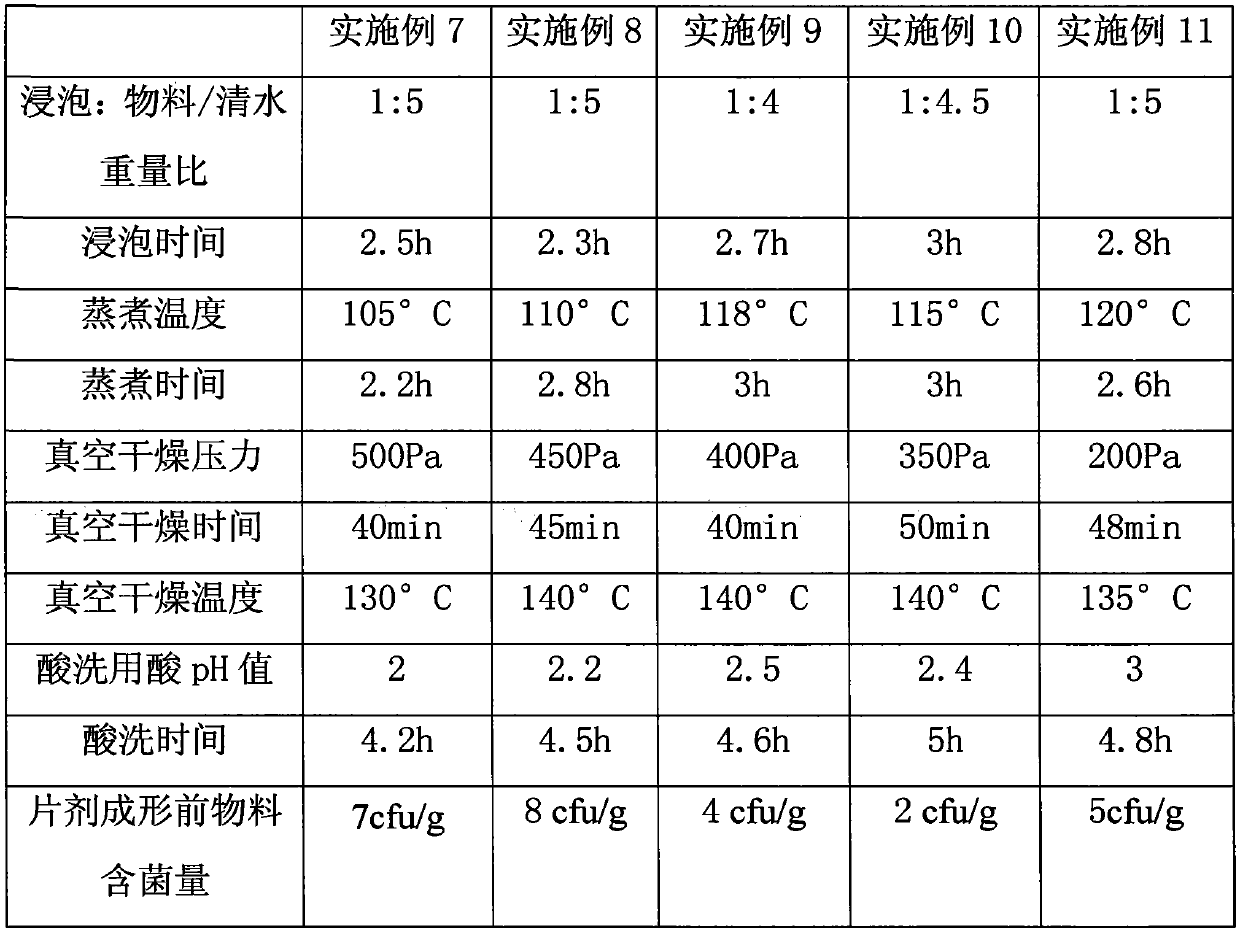
[0037] Examples 7-11 Refer to Table 2 for the corresponding parameters of each step.
[0038]
[0039] Table 2
[0040] It can be seen from the above table that the vacuum drying pressure and the vacuum drying temperature have a significant impact on the bacterial content, and the active ingredient content of Hijiki in Examples 7-11 is slightly lower than that in Examples 2-6.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com