Suppository used for treating cancer
A technology for suppositories and cervical cancer, applied in the application of drugs, in the field of suppositories, can solve the problems of limited drug concentration, toxic and side effects, and low bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
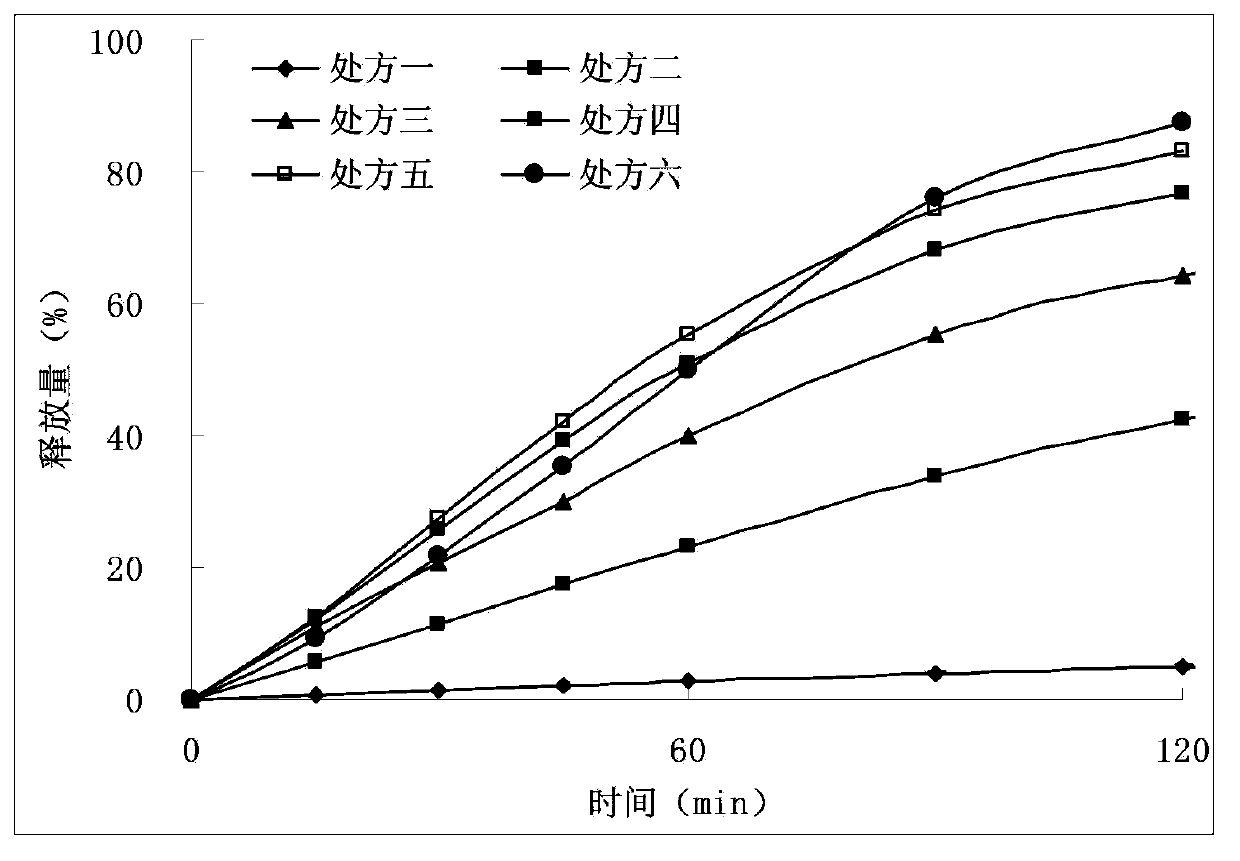
Image
Examples
experiment example 1
[0052] In vitro killing activity of temozolomide API on tumor cells
[0053] Materials and Methods
[0054] Test substance: temozolomide, Tianjin Tasly Group Co., Ltd.
[0055] Positive control drug: temozolomide (raw material drug), Jiangsu Tasly Diyi Pharmaceutical Co., Ltd.
[0056] Drug preparation method: before use, weigh the raw material drug powder, dissolve it with dimethyl sulfoxide (DMSO), and dilute it with cell culture medium to the concentration required for the experiment.
[0057] Cell lines: Human cervical cancer cells ME180 and MS751 were obtained from ATCC. Human colon cancer cell line HT-29 and human cervical cancer cell line Hela are all cell lines preserved in our laboratory.
[0058] Reagents: RPMI1640 is a product of GIBCO; MTT is a product of Bebco; calf serum was purchased from Hangzhou Sijiqing Bioengineering Materials Co., Ltd.
[0059] Instrument: BIORAD550 microplate reader.
[0060] Experimental method: tetrazolium salt [3-(4,5-dimethylthiaz...
experiment example 2
[0076] Inhibitory effect of temozolomide smear on human cervical carcinoma MS751 subcutaneously transplanted tumor in nude mice
[0077] Basic Experimental Process
[0078] Tumor-bearing animals with good tumor growth and good general condition were selected and sacrificed by cervical dislocation. The tumor mass was taken out under aseptic conditions, cut into a tumor mass with a diameter of 2-3 mm with a scalpel, and inoculated subcutaneously in the axilla of nude mice with a trocar. Or grind the tumor tissue and pass it through a 100-mesh sieve to prepare a cell suspension in a volume of 3×10 7 / 0.1ml / only inoculated subcutaneously in nude mice. A few days later, the average volume of tumors in tumor-bearing mice reached 100mm 3 In the above cases, the animals were randomly divided into stratified groups according to the tumor volume, and the animals in each group were given drugs according to the experimental plan. Wherein the negative control group tumor grows naturall...
experiment example 3
[0117] Inhibitory effect of temozolomide smear on human cervical cancer HELA subcutaneous xenografts
[0118] Basic experimental method is the same as experimental example 2
[0119] Materials and Methods
[0120] Experimental sample: temozolomide ointment, the content is 2.5%, 5.0%, 10.0%
[0121] Control drug: doxorubicin hydrochloride (doxorubicin) for injection, a product of Pharmacia Italia S.p.A, converted to the intraperitoneal injection of the animal according to the drug instruction manual
[0122] Animals: BALB / c nu mice, female, 5-6 weeks old
[0123] Model: nude mice bearing cervical cancer HELA tumor
[0124] experiment procedure:
[0125] Seven days after the inoculation of the tumor block, the tumor volume of the tumor-bearing mice reached an average of 100 mm 3 Above, according to tumor volume size random stratification grouping. A negative control group was set up; temozolomide 2.5%, 5% and 10% three content groups and a blank dosage form control group; ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com