Supramolecular hybrid peptide dendric macromolecule self-assembly and preparation method and applications thereof
A technology of dendrimers and supramolecules, applied in other methods of inserting foreign genetic materials, preparations for in vivo experiments, medical preparations of non-active ingredients, etc., can solve the problem of limited and increased peptide dendrimers Problems such as production cost and production difficulty, limited number of functional groups, etc., to achieve the effects of amplifying highly branched structures, green and efficient functional integration, and optimizing characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
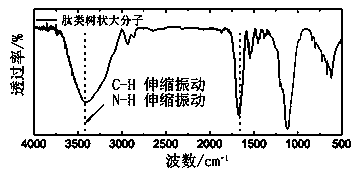
[0071] Example 1: Preparation of Peptide Dendrimers
[0072] a) Protect the functional group of the amino acid: protect the amino acid according to the difference of the surface functional group of the core molecule of the peptide dendrimer to be prepared. If the surface functional group of the core molecule is an amino group, protect the amino group of the amino acid. If it is a hydroxyl or carboxyl group, the carboxyl group of the amino acid is protected;
[0073] b) Preparation of a first-generation dendrimer: Weigh the core molecule (functionality is n, n=1 or 2), amino acid with protective group (1.5n equivalent), condensing agent (1.5n equivalent), catalyst ( 1.5n equivalent) and organic base (4n equivalent), at 0°C, under the condition of nitrogen protection, add anhydrous solvent for dehydration condensation reaction; then react at room temperature, after the reaction, the resulting solution is washed, dried, and concentrated under reduced pressure , separated by co...
Embodiment 2
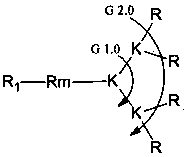
[0079] Example 2: Concrete synthetic examples of peptide dendrimers (synthetic route such as figure 1 )
[0080] 1. Modification of core molecules.
[0081] Lipoic Acid Derivatives (LA-NH 2 )Synthesis
[0082]Weigh 5.0 g of lipoic acid (LA), 4.6 g of mono-tert-butoxycarbonyl-protected ethylenediamine, 6.9 g of 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDCI) and 4.9 g of 1-hydroxybenzotriazole (HOBT) were added to a 100 mL single-neck bottle with a branch tube, vacuumed and filled with nitrogen. Add 40 mL of redistilled dichloromethane (DCM) with a syringe, add 15.7 mL of N,N-diisopropylethylamine (DIPEA) while stirring in an ice bath, continue stirring for half an hour, remove the ice bath, and react at room temperature for 24 h. After the solvent was removed, chloroform was added to dissolve, followed by saturated NaHCO 3 , NaHSO 4 , NaCl wash, with anhydrous MgSO 4 After drying, filter, spin off the solvent, and separate by column chromatograph...
Embodiment 3
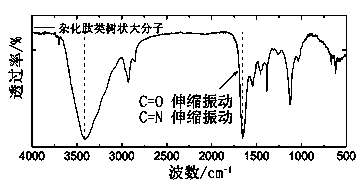
[0096] Example 3: Preparation of Supramolecular Hybrid Peptide Dendrimers
[0097] The synthesis of supramolecular hybrid peptide dendrimers can be arbitrarily selected from the following two methods:
[0098] method one:
[0099] ① Reduction of peptide dendrimers. Dissolve the peptide dendrimers in the mixed solvent of ethyl acetate and water, add the same amount of NaBH 4 , after stirring for 1 h, the solution was diluted with water and extracted three times with chloroform, the organic phases were combined, anhydrous MgSO 4 After drying, filtering and spin-off of solvent, the crude product was separated by column chromatography to obtain reduced peptide dendrimers.
[0100] ②Coordination assembly into supramolecular hybrid peptide dendrimers. Disperse the inorganic nanoparticles in the organic phase, add a large excess of the salt solution of the reduced peptide dendrimers, react at room temperature for 30 minutes, raise the temperature to 60°C and react overnight to...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com