Compound inhalation preparation containing penicillin antibiotic and glucocorticoid
A technology of glucocorticoid and penicillin, applied in the field of inhalation preparations, can solve the problems of inconvenience, poor curative effect, easy decomposition and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
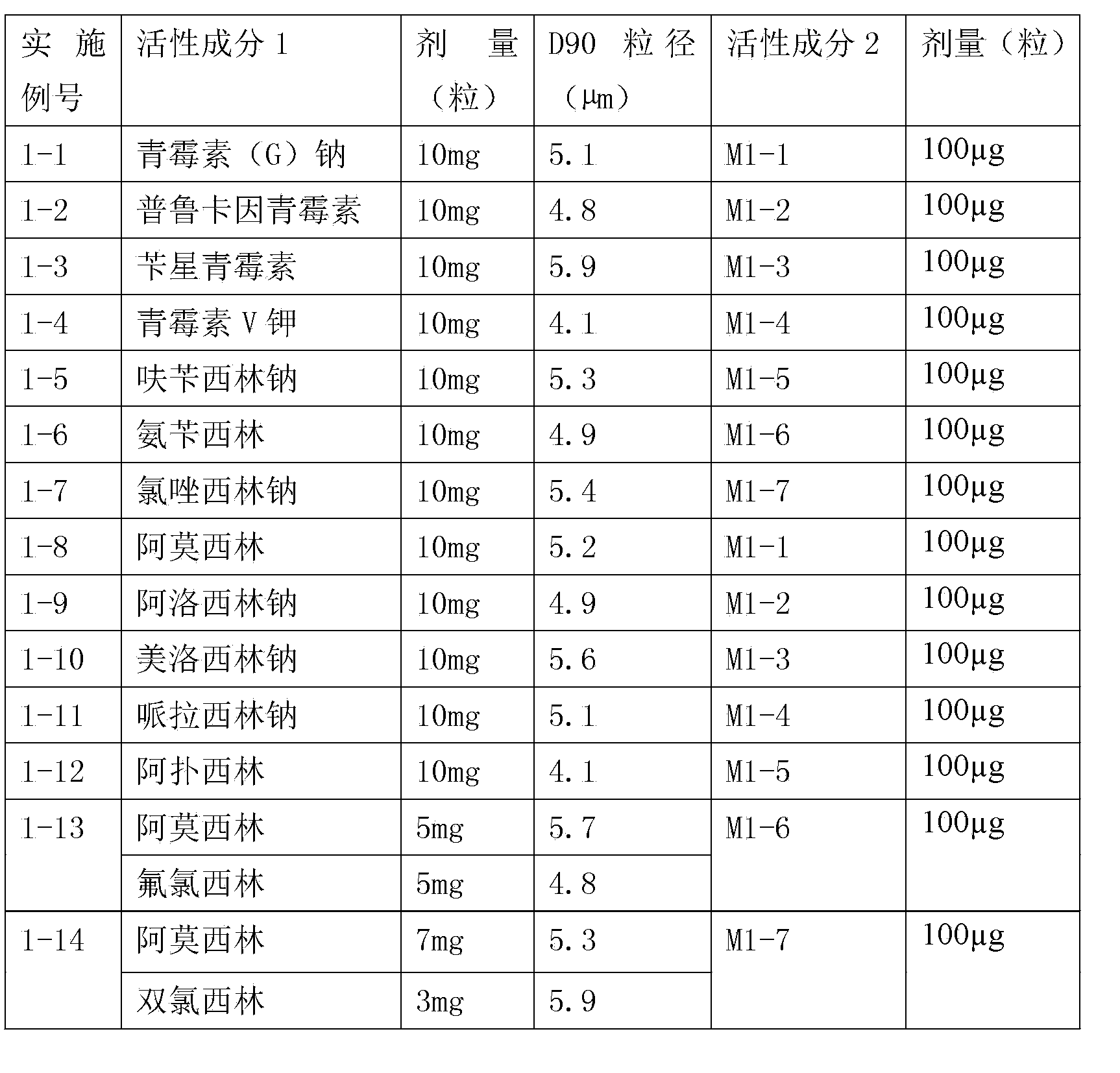
Embodiment 1
[0044] According to the following table, the active ingredient is micronized with a flow energy mill. The particle size of D90 is shown in the table below. 8g of anhydrous lactose is micronized with a flow energy mill to an average particle size of 36μm. In 1000 capsules size 3, the active ingredients in each capsule are listed in the table below.
[0045]
Embodiment 2
[0047] According to the table below, the active ingredient is micronized with a flow energy mill. The particle size of D90 is shown in the table below. 8g of anhydrous lactose is micronized with a flow energy mill to an average particle size of 40μm. In 1000 capsules size 3, the active ingredients in each capsule are listed in the table below.
[0048]
Embodiment 3
[0050] According to the table below, the active ingredient is micronized with a flow energy mill. The particle size of D90 is shown in the table below. 8g of anhydrous lactose is micronized with a flow energy mill to an average particle size of 42μm. In 1000 capsules size 3, the active ingredients in each capsule are listed in the table below.
[0051]
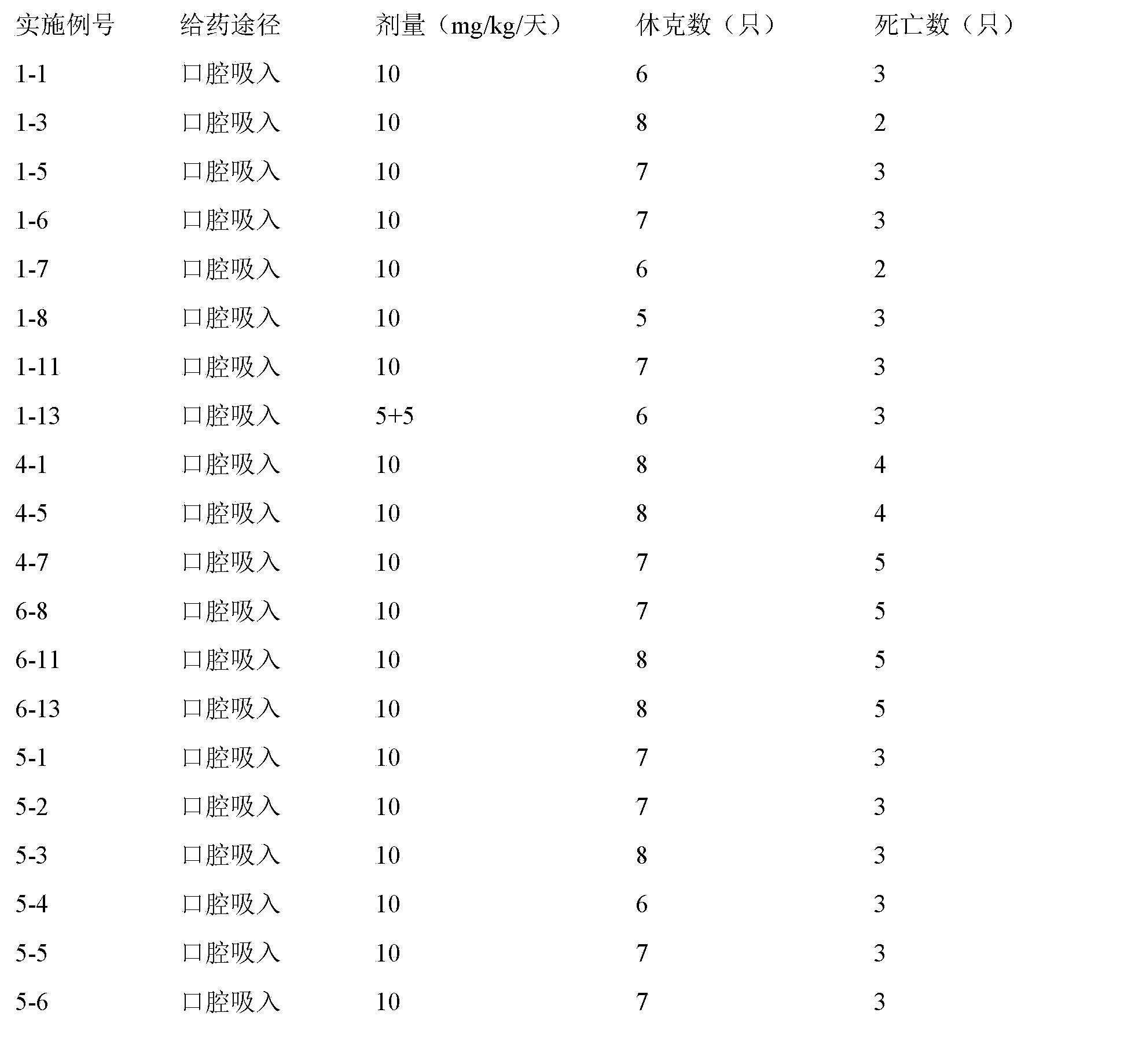
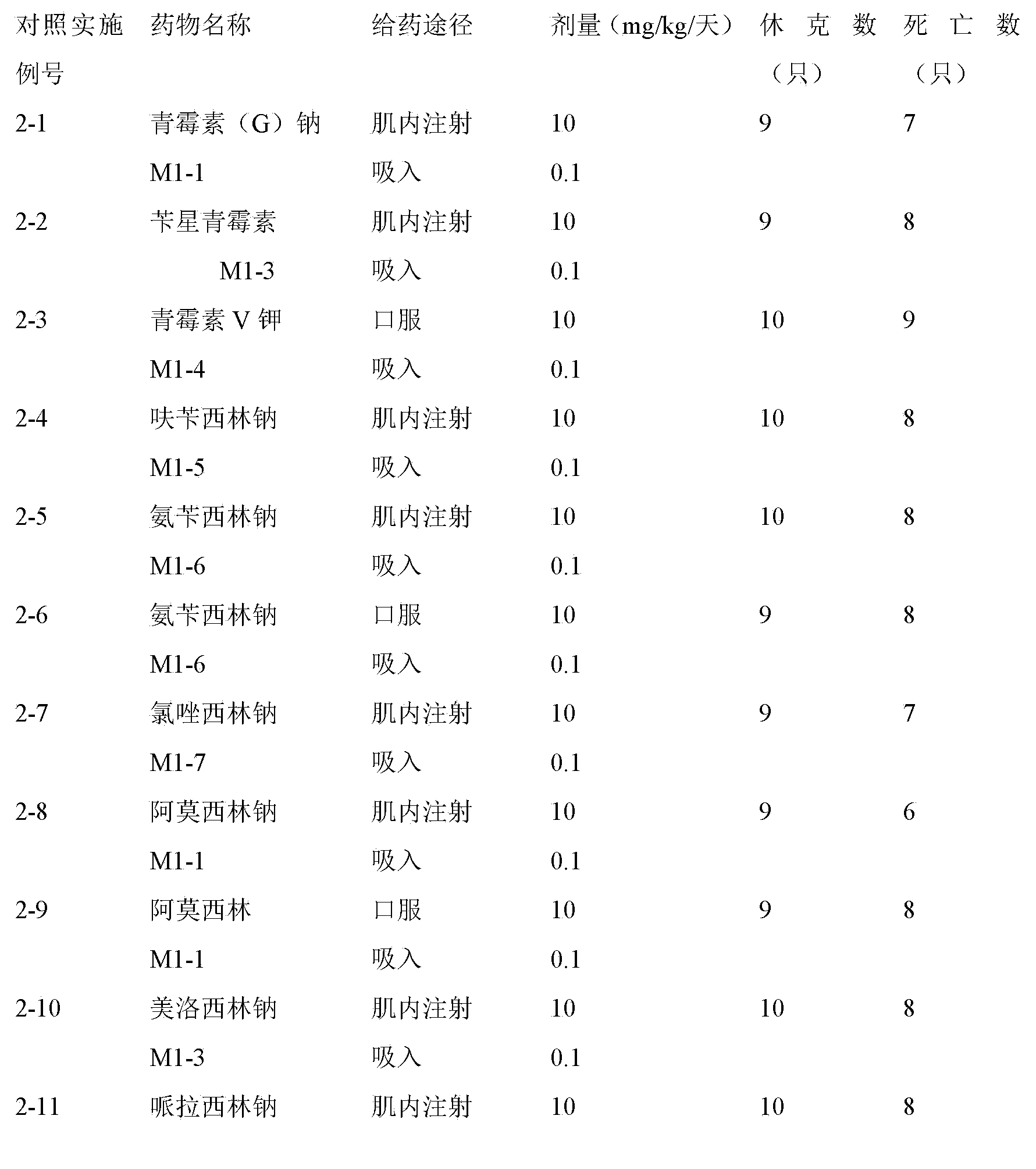
[0052] The preparation of aerosol, the active ingredient used is the weight of 1000 dosage units, HFA-134a is 1,1,1,2-tetrafluoroethane, HFA-227 is 1,1,1,2,3,3, 3-Heptafluoropropane.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com