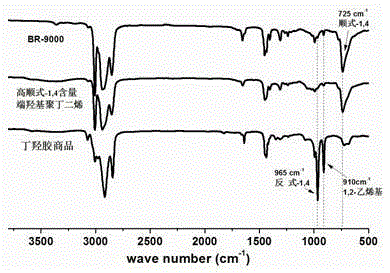
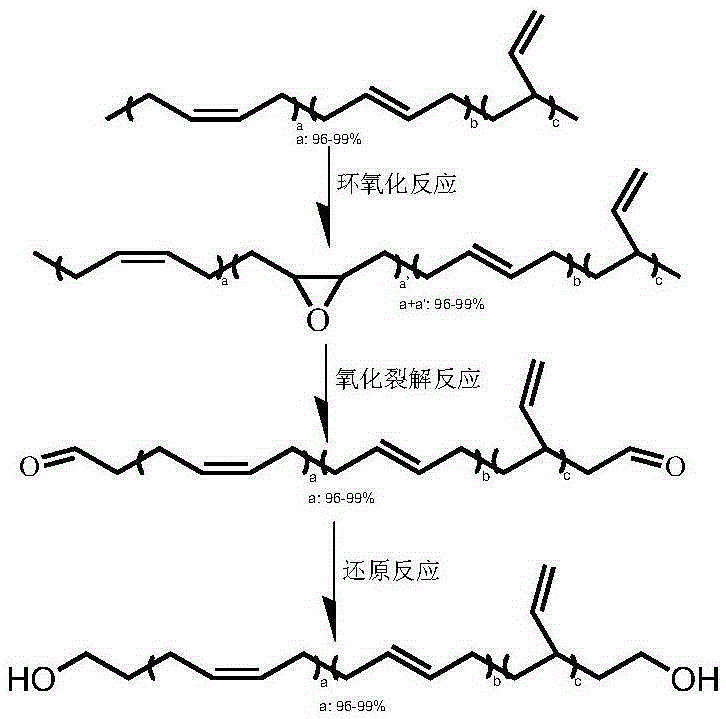
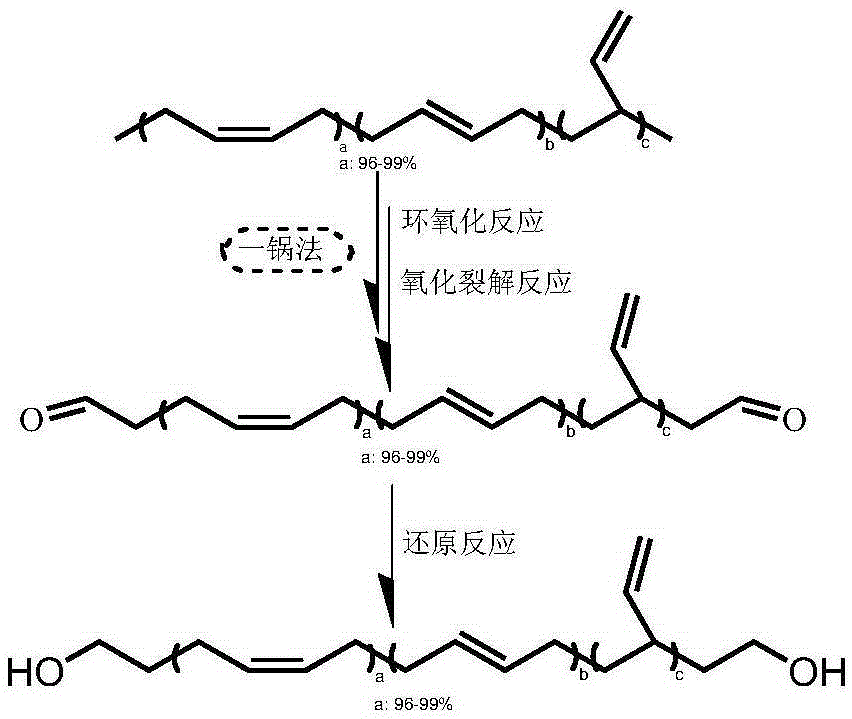
High cis-1,4 content hydroxyl terminated polybutadiene liquid rubber and preparation method thereof
A technology of hydroxyl-terminated polybutadiene and aldehyde-terminated polybutadiene, applied in the field of high-cis-1,4-content hydroxyl-terminated polybutadiene liquid rubber and its preparation, can solve the problem of difficult to achieve hydroxyl-terminated polybutadiene Butadiene microstructure, can not be prepared and other problems, to achieve high reaction efficiency, the effect of a wide range of industrial application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Take 5.00g of nickel-based butadiene rubber (BR9000), dissolve it completely in 100mL of tetrahydrofuran, the rubber concentration is 50g / L, keep the temperature at 30°C, add m-chloroperoxybenzoic acid under vigorous stirring, the amount of m-chloroperoxybenzoic acid is The butadiene structural unit in nickel-based butadiene rubber (BR9000) is 2%, the reaction time is 6 hours, and the product is precipitated with a large amount of ethanol and vacuum-dried. product by 1 HNMR showed that the epoxy rate was 2.2%.
[0052] Take 4.00g of the above-mentioned epoxidized butadiene rubber, completely dissolve it in 100mL of tetrahydrofuran, the rubber concentration is 40g / L, keep the temperature at 30°C, add periodic acid under vigorous stirring, the molar ratio of periodic acid to epoxy group is 1 : 1, reacted for 30 minutes. The product can be filtered to obtain polybutadiene liquid rubber with high cis-1,4 content and terminal aldehyde group.
[0053] Take 3.00 g of the ab...
Embodiment 2
[0055] Take 5.00g of cobalt-based butadiene rubber (Taipol), dissolve it completely in 200mL of cyclohexane, the rubber concentration is 25g / L, keep the temperature at 50°C, add m-chloroperoxybenzoic acid and m-chloroperoxybenzoic acid under vigorous stirring The dosage is 4% of the butadiene structural unit in cobalt-based butadiene rubber (Taipol), the reaction time is 4 hours, and the product is precipitated with a large amount of ethanol and dried in vacuum. product by 1 HNMR showed that the epoxy rate was 4.1%.
[0056] Take 4.00g of the above-mentioned epoxidized butadiene rubber, completely dissolve it in 100mL cyclohexane, the rubber concentration is 40g / L, keep the temperature at 50°C, add periodic acid under vigorous stirring, the molar ratio of periodic acid to epoxy group 1:1, reaction for 40 minutes. The product can be filtered to obtain polybutadiene liquid rubber with high cis-1,4 content and terminal aldehyde group.
[0057] Take 3.00 g of the above-mentione...
Embodiment 3
[0059]Take 5.00g of neodymium-based butadiene rubber (BR9100), dissolve it completely in 100mL of toluene, the rubber concentration is 50g / L, keep the temperature at 70°C, and add (1S,2S)-(+)-[1,2- Cyclohexanediamine nitrogen-N,N'-bis(3,5-di-tert-butyl salicylidene)] manganese(III) chloride 0.005g, the amount of sodium hypochlorite is in neodymium-based butadiene rubber (BR9100) 8% butadiene structural unit, the reaction time is 2 hours, the product is precipitated with a large amount of ethanol and dried in vacuum. product by 1 HNMR showed that the epoxy rate was 8.1%.
[0060] Take 4.00g of the above epoxidized butadiene rubber, completely dissolve it in 100mL of toluene, the rubber concentration is 40g / L, keep the temperature at 50°C, add periodic acid under vigorous stirring, the molar ratio of periodic acid to epoxy group is 1 : 1, reacted for 50 minutes. The product can be filtered to obtain polybutadiene liquid rubber with high cis-1,4 content and terminal aldehyde g...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| Functional group degree | aaaaa | aaaaa |
| epoxy rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com