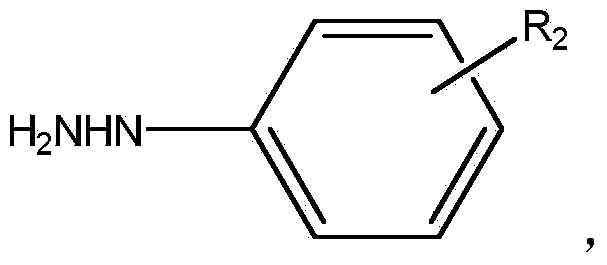
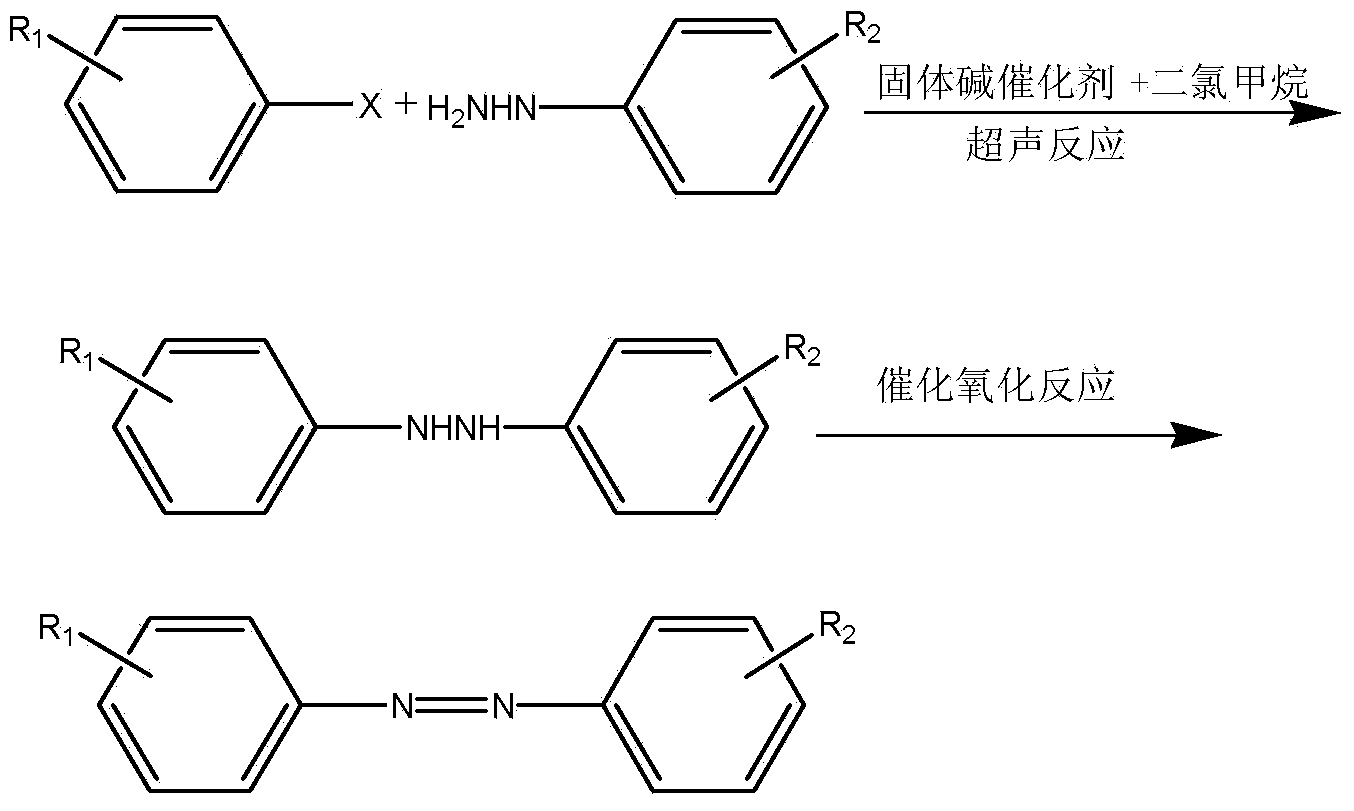
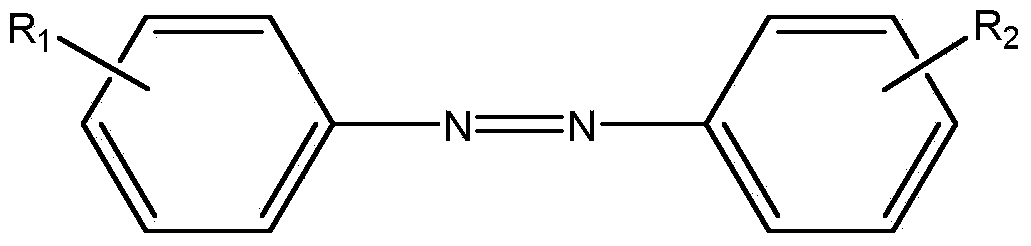Preparation method of asymmetric aromatic azo compound
An aromatic azo and asymmetric technology, applied in the field of preparation of asymmetric aromatic azo compounds, can solve the problems of high operation requirements, easy environmental pollution, poor selectivity, etc., and achieves good selectivity, safe and simple operation, and reduced processing intensity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Preparation of 4-nitrophenylazobenzene:
[0034] The structural formula is:
[0035]
[0036] Preparation process: Add 1.0mol phenylhydrazine (108.1g), 500ml dichloromethane, and 8g potassium carbonate-modified alumina solid base to a reaction vessel equipped with stirring and a thermometer in sequence, control the temperature at 20-40°C, and add dropwise 1.05mol of 4-nitrochlorobenzene (165.4g), after dropping, place the reaction vessel under ultrasonic conditions at 38-40°C for 1 hour, filter to remove the solid, add 200ml of 10wt% sodium hypochlorite solution to the filtrate, and stir at room temperature for reaction After 6 hours, the layers were left to stand, and the water phase was removed. The dichloromethane layer was concentrated again to obtain the crude product, and the crude product was recrystallized with dichloromethane to obtain 196g of 4-nitrophenylazobenzene with a purity of 98.8% (total yield 86.3%), the recovered dichloromethane can be recycled a...
Embodiment 2
[0038] Preparation of 4-nitrophenylazo 4-methoxybenzene:
[0039] The structural formula is:
[0040]
[0041] Preparation process: Add 1.0mol 4-nitrophenylhydrazine (153.1g), 1000ml dichloromethane, and 20g potassium carbonate-modified alumina solid base to a reaction vessel equipped with stirring and a thermometer, and control the temperature at 20-40°C , add 1.10mol of 4-methoxybromobenzene (205.7g) dropwise, after dropping, place the reaction bottle under ultrasonic conditions at 38-40°C for 2 hours, filter to remove the solid, add 200ml of 10wt% sodium hypochlorite solution to the filtrate , stirring and reacting at room temperature for 5 hours, standing to separate layers, removing the water phase, and then concentrating the dichloromethane layer to obtain a crude product, which was recrystallized with dichloromethane to obtain 4-nitrophenylazo 4- Methoxybenzene 225g (total yield 87.5%), the dichloromethane recovered can be recycled after treatment.
Embodiment 3
[0043] Preparation of 2,4-dinitrophenylazo 4-nitrobenzene:
[0044] The structural formula is:
[0045]
[0046] Preparation process: Add 1.0mol 2,4-dinitrophenylhydrazine (198.2g), 1500ml dichloromethane, and 20g potassium nitrate-modified alumina solid base to a reaction vessel equipped with stirring and a thermometer, and control the temperature at 20 -40°C, add 1.20mol of 4-nitrochlorobenzene (189.0g) dropwise, after dropping, place the reaction bottle under ultrasonic conditions at 38-40°C for 1.5 hours, filter to remove the solid, add 300ml of filtrate, 25% Hydrogen peroxide, stirred and reacted at room temperature for 4 hours, let stand to separate layers, removed the water phase, concentrated the dichloromethane layer to obtain the crude product, and recrystallized the crude product with dichloromethane to obtain 4-nitrophenylazobenzene with a purity of 99.3% 288g (total yield 90.8%), the recovered dichloromethane can be recycled after treatment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com