A mitochondria-targeted iridium-n-heterocyclic carbene complex and its preparation method and application
A technology of heterocyclic carbene and mitochondria, which is applied in the direction of medical preparations containing active ingredients, pharmaceutical formulas, and compounds containing elements of Group 8/9/10/18 of the periodic table, etc., can solve problems such as differences in functional activities, and achieve The effect of increasing water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] 1. Preparation of complex Ir-1
[0046] (1) Preparation of Ligand L1:
[0047] Add dichloromethane (1.699 g, 20 mmol) and 1-methylimidazole (4.926 g, 60 mmol) into the reaction flask, and react in a closed container at 75 °C for 24 h. After the reaction, cool to room temperature and spin After the solvent was evaporated to dryness, it was dissolved with a small amount of methanol, added to a large amount of tetrahydrofuran and stirred at room temperature for 2 h, a large amount of white solid was formed, filtered, washed with tetrahydrofuran, and dried in vacuum to obtain a white solid to obtain 2.123 g of the ligand, with a yield of 42.6%.
[0048] 1 H NMR (300 MHz, DMSO) δ 9.86 (s, 2H), 8.27 (s, 2H), 7.79 (s, 2H), 6.89 (s, 2H), 3.88 (s, 6H). 13 C NMR (75 MHz, DMSO) δ 138.69, 124.60, 122.53, 57.99, 36.66.
[0049] ESI-MS: Theoretical: m / z 177.2 [M-Cl-H] + and 89.1 [M-2Cl] + ;Experimental value: m / z [M-Cl-H] + 177.1 and 89.0 [M-2Cl] + .
[0050] Elemental Anal...
Embodiment 2
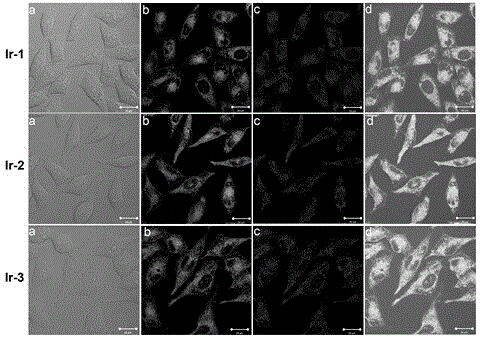
[0078] Example 2 Mitochondrial targeting and mitochondrial swelling induced by iridium-N-heterocyclic carbene complexes
[0079] 1. Measure the mitochondrial targeting and mitochondrial swelling tracking of the iridium-N-heterocyclic carbene complex prepared by the present invention by laser confocal fluorescence microscopy. The assay method is as follows:
[0080] The cells were digested with 0.25% trypsin into a single-cell suspension, and the number of viable cells was recorded using a hemocytometer. About 200,000 cells per well were seeded in a confocal culture dish, and after 24 hours of culture, mitochondrial probes were added Mito-tracker Red (purchased from Invitrogen) and 20 μM iridium-N-heterocyclic carbene complex, at 37 °C, in 5% (volume concentration) CO 2 Incubated in an incubator for 30 min. Then suck up and discard the old medium, wash twice with phosphate buffered saline (PBS), add 1 mL of PBS, and immediately observe under a confocal laser microscope.
[00...
Embodiment 3
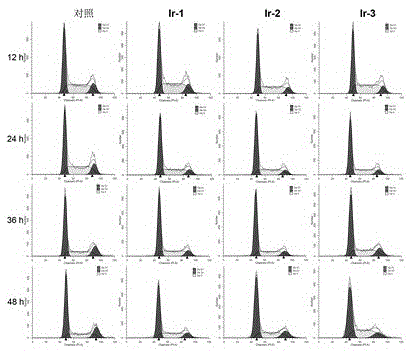
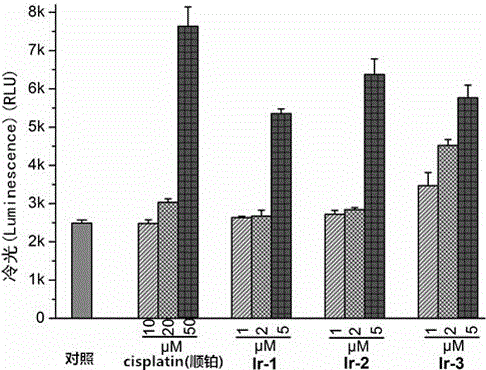
[0082] Example 3 Antitumor Activity and Phototoxicity to Tumor Cells of Iridium-N-Heterocyclic Carbene Complexes
[0083] 1. In this example, the antitumor activity of the iridium-N-heterocyclic carbene complexes prepared in the present invention is evaluated, and the drug cisplatin is used as a control, and the grouping conditions are as follows:
[0084] Control group: drug cisplatin (cisplatin); experimental group: iridium-N-heterocyclic carbene complex.
[0085] 2. Cytotoxicity was determined by tetrazolium salt (MTT) colorimetric method, the specific determination method is as follows:
[0086] The cells were digested with trypsin with a mass volume ratio of 0.25% into a single cell suspension, and the number of viable cells was recorded using a hemocytometer, and the concentration of viable cells was adjusted to 5×10 4 / mL, inoculated in a 96-well culture plate, 160 μL per well, after 24 hours of culture, then added different concentrations of drugs, placed at 37 ℃, in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com