Method for synthesizing quantum dot-polypeptide compound with peptoid connecting arm
A technique for polypeptide complexes and synthetic methods, applied in the field of quantum dot-peptide complexes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Example 1: Synthesis of porcine enterotoxin-derived Escherichia coli K88ac adhesion antigen-8 unit thiazole side chain peptoid linker-quantum dot complex:
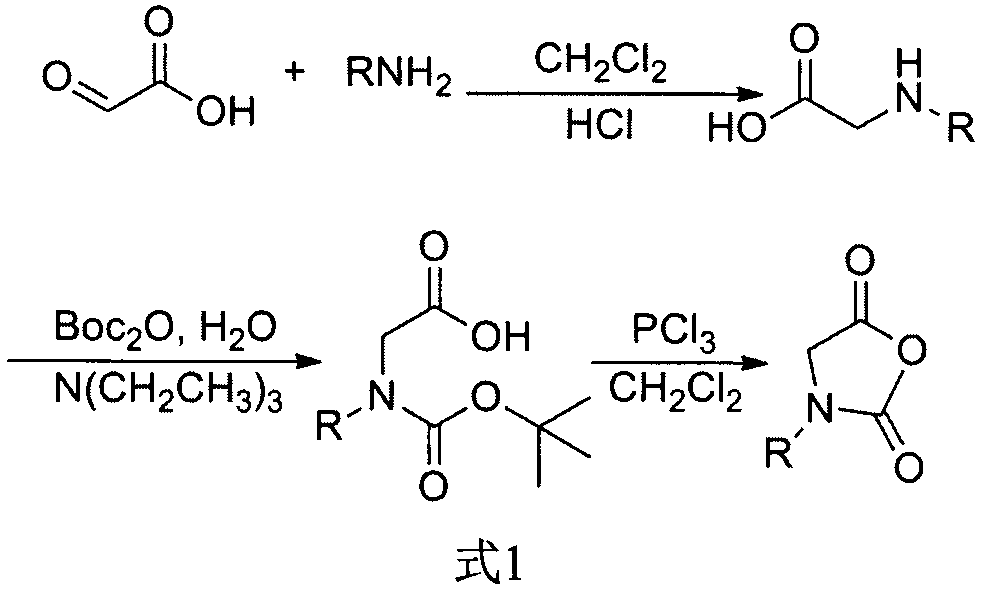
[0016] 1. Synthesis of N-thiazole-N-internal carboxylic acid anhydride
[0017] Glyoxylic acid (14.9g) and aminothiazole (3.4g) were weighed, dissolved in dichloromethane (250mL), stirred at room temperature for 18 hours, the solvent was evaporated, and hydrochloric acid solution (1M, 250mL) was added. After the mixture was heated under reflux for 18 hours, the solvent was removed by evaporation to obtain 12.3 g of off-white solid, acetaldehyde amine, with a yield of 83%. Weigh acetaldehyde amine (8.62g), di-tert-butyl dicarbonate (28.1g), triethylamine (35.8mL), blend and dissolve in distilled water (200mL), stir at room temperature for 18 hours, add n-hexane (100mL ) extraction 3 times. The aqueous phase was blended with hydrochloric acid solution (4M, 50 mL), and extracted 5 times with ethyl acetate (100 mL). ...
Embodiment 2
[0020] Example 2: Synthesis of hepatitis B virus core antigen-12 unit imidazoline side chain peptoid linker-quantum dot complex:
[0021] 1. Synthesis of N-imidazoline-N-internal carboxylic acid anhydride
[0022]Glyoxylic acid (14.9g) and aminoimidazole (2.9g) were weighed, dissolved in dichloromethane (250mL), stirred at room temperature for 18 hours, the solvent was evaporated, and hydrochloric acid solution (4M, 100mL) was added. After the mixture was heated under reflux for 36 hours, the solvent was removed by evaporation to obtain 12.1 g of acetaldehyde amine as an off-white solid, with a yield of 86%. Weigh acetaldehyde amine (7.44g), di-tert-butyl dicarbonate (27.2g), triethylamine (31.0mL), dissolve in distilled water (200mL), stir at room temperature for 18 hours, extract with n-hexane (100mL) for 3 Second-rate. The aqueous phase was blended with hydrochloric acid solution (4M, 50 mL), and extracted 5 times with ethyl acetate (100 mL). The organic phase was extrac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com