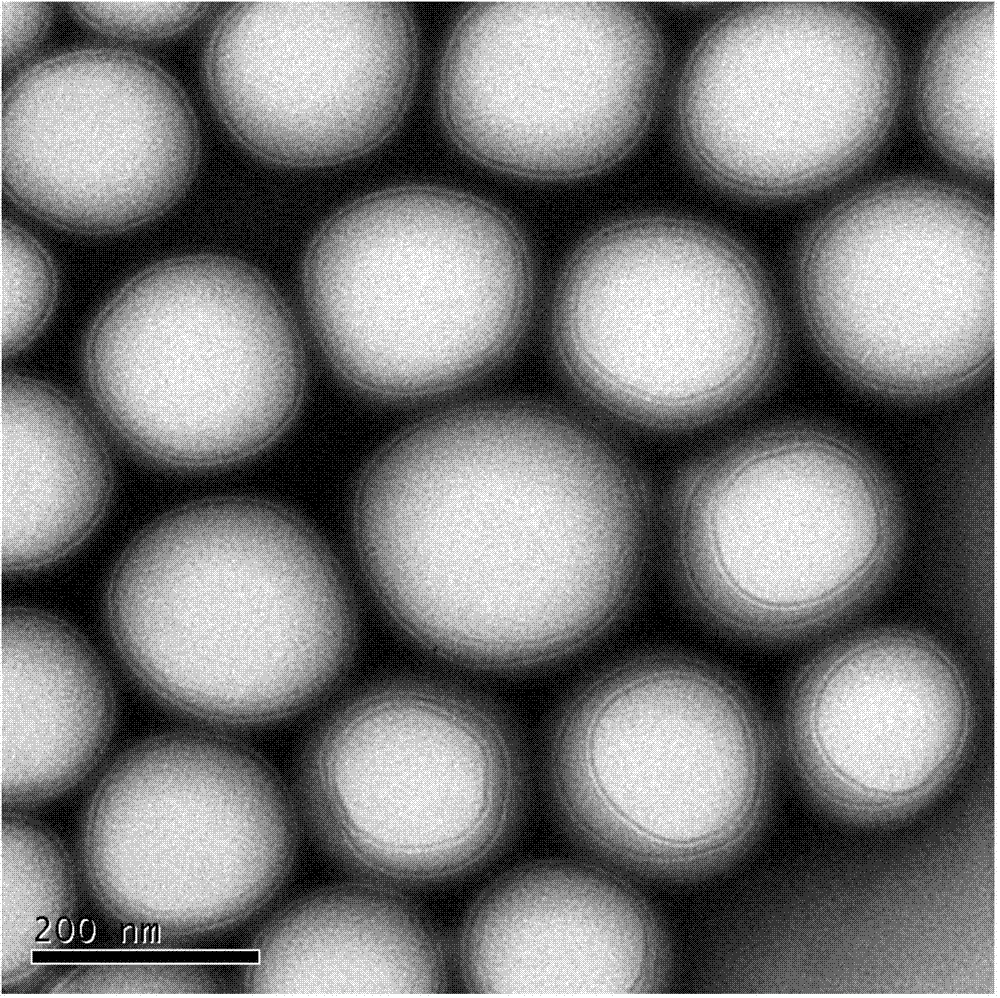
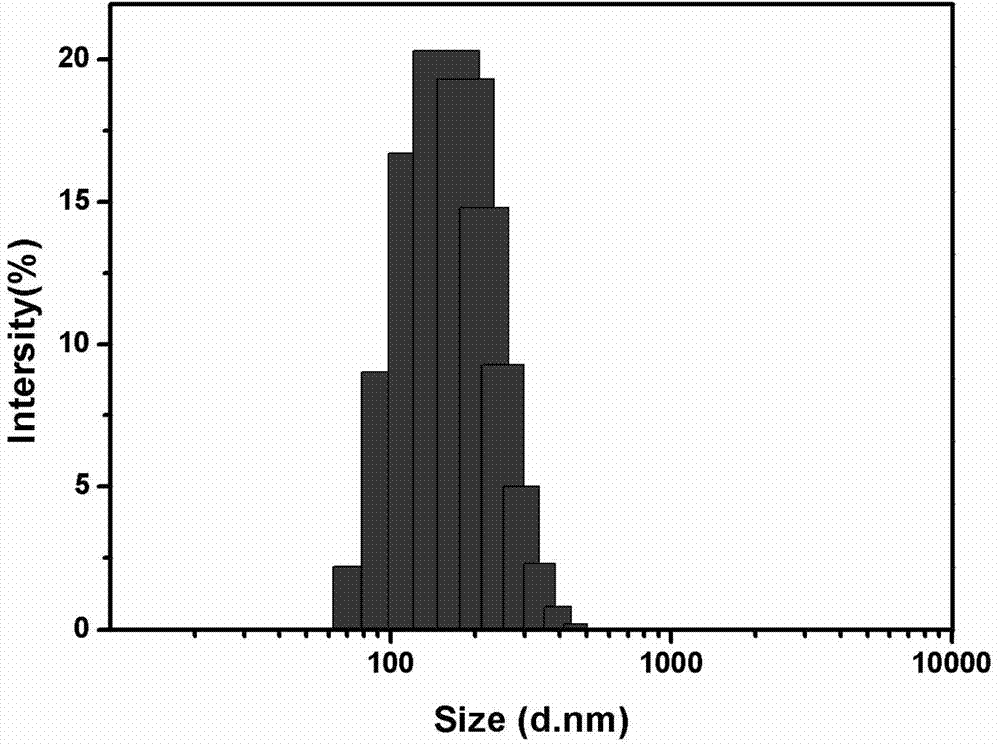
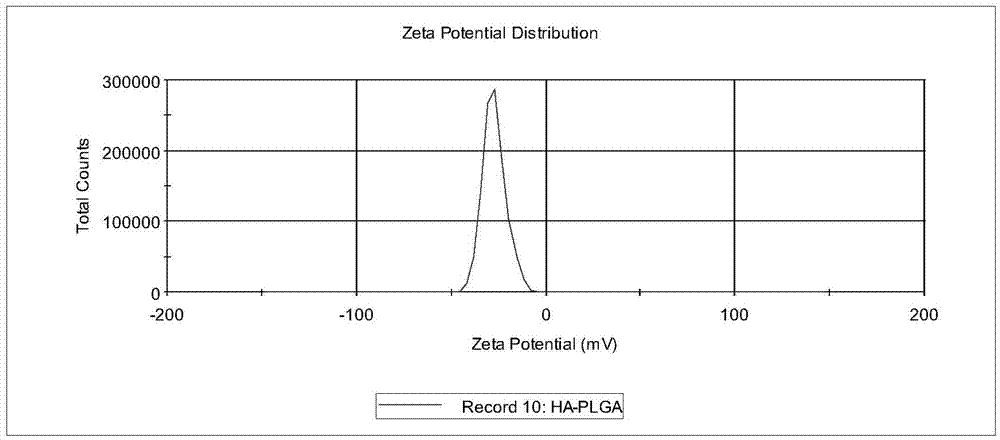
Hyaluronic acid-cystamine-polylactic acid-glycollic acid graft polymer and preparation method thereof
A graft polymer, glycolic acid technology, applied in the field of hyaluronic acid-cystamine-polylactic acid-glycolic acid graft polymer and its preparation, can solve the problems of strong hydrophobicity of aliphatic polyester and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] (a) Combine 5g of polylactic acid-glycolic acid (molar ratio of lactic acid to glycolic acid 75 / 25, molecular weight: 20,000, Jinan Daigang), 0.144g of 1-ethyl-(3-dimethylaminopropyl ) Carbodiimide (Alfar Aesar, 98%) and 0.086g of N-hydroxysuccinimide (Alfar Aesar, 98%) were placed in a three-necked flask. After vacuuming for 1 hour, 15 mL of dichloromethane was added Dissolve, at a temperature of 20 DEG C, and react for 2 hours under nitrogen protection to obtain a crude product of N-hydroxysuccinimide activated polylactic acid-glycolic acid. The crude product solution was rotary evaporated at 25°C to remove dichloromethane, and then precipitated with 200mL ether, washed with a methanol / ether (3:7) mixed solution (100mL×3 times), and dried in a vacuum oven at 25°C for 36 hours To obtain 4.245 g of N-hydroxysuccinimide polylactic acid-glycolic acid as a solid product.
[0093] (b) Add 4.245 g of polylactic acid-glycolic acid activated by N-hydroxysuccinimide to 20 mL of d...
Embodiment 2
[0107] (a) Combine 5g of polylactic acid-glycolic acid (mole ratio of lactic acid to glycolic acid 75 / 25, molecular weight: 50,000, Jinan Daigang), 0.115g of 1-ethyl-(3-dimethylaminopropyl ) Carbodiimide (Alfar Aesar Company, 98%) and 0.081g of 1-hydroxybenzotriazole (Aladdin Reagent Shanghai Co., Ltd., 99%) were placed in a three-necked flask. After vacuuming for 1 hour, 10 mL of The chloroform is dissolved, the temperature is 40 DEG C, and the reaction is carried out for 4 hours under the protection of nitrogen to obtain the crude product of 1-hydroxybenzotriazole activated polylactic acid-glycolic acid. The crude product solution was rotary evaporated at 25°C to remove chloroform, and then precipitated with 200mL ether, washed with a methanol / ether (5:5) mixed solution (100mL×3 times), dried in a vacuum oven at 25°C for 36 hours to obtain The solid product 1-hydroxybenzotriazole activated polylactic acid-glycolic acid 4.461g.
[0108] (b) Add 4.461 g of 1-hydroxybenzotriazole...
Embodiment 3
[0114] (a) 5g of polylactic acid-glycolic acid (mole ratio of lactic acid to glycolic acid is 50 / 50, molecular weight: 20,000, Jinan Daigang), 0.309g of N,N-dicyclohexylcarbodiimide (Sinopharm Group Chemical Reagent Co., Ltd., 99%) and 0.173g of N-hydroxysuccinimide (Alfar Aesar, 98%) were placed in a three-necked flask. After vacuuming for 1 hour, add 50mL of dichloromethane to dissolve at a temperature of 25 It was reacted for 4 hours under the protection of nitrogen at ℃ to obtain the crude product of N-hydroxysuccinimide activated polylactic acid-glycolic acid. Then it was precipitated with 200mL ether, washed with methanol / ether (3:7) mixed solution (100mL×3 times), dried in a vacuum oven at 25°C for 36 hours to obtain a solid product of N-hydroxysuccinimide polylactic acid- Glycolic acid 4.845g.
[0115] (b) Add 4.845 g of N-hydroxysuccinimated polylactic acid-glycolic acid to 30 mL of dimethyl sulfoxide to prepare an N-hydroxysuccinimated polylactic acid-glycolic acid dim...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Weight average molecular weight | aaaaa | aaaaa |
| Weight average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com