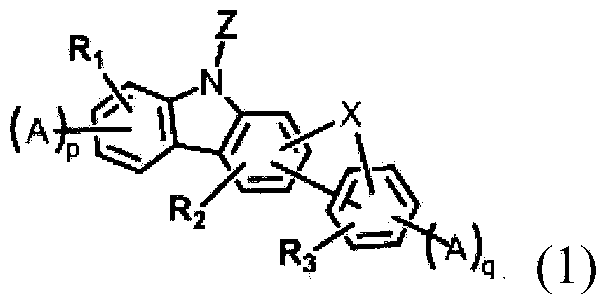
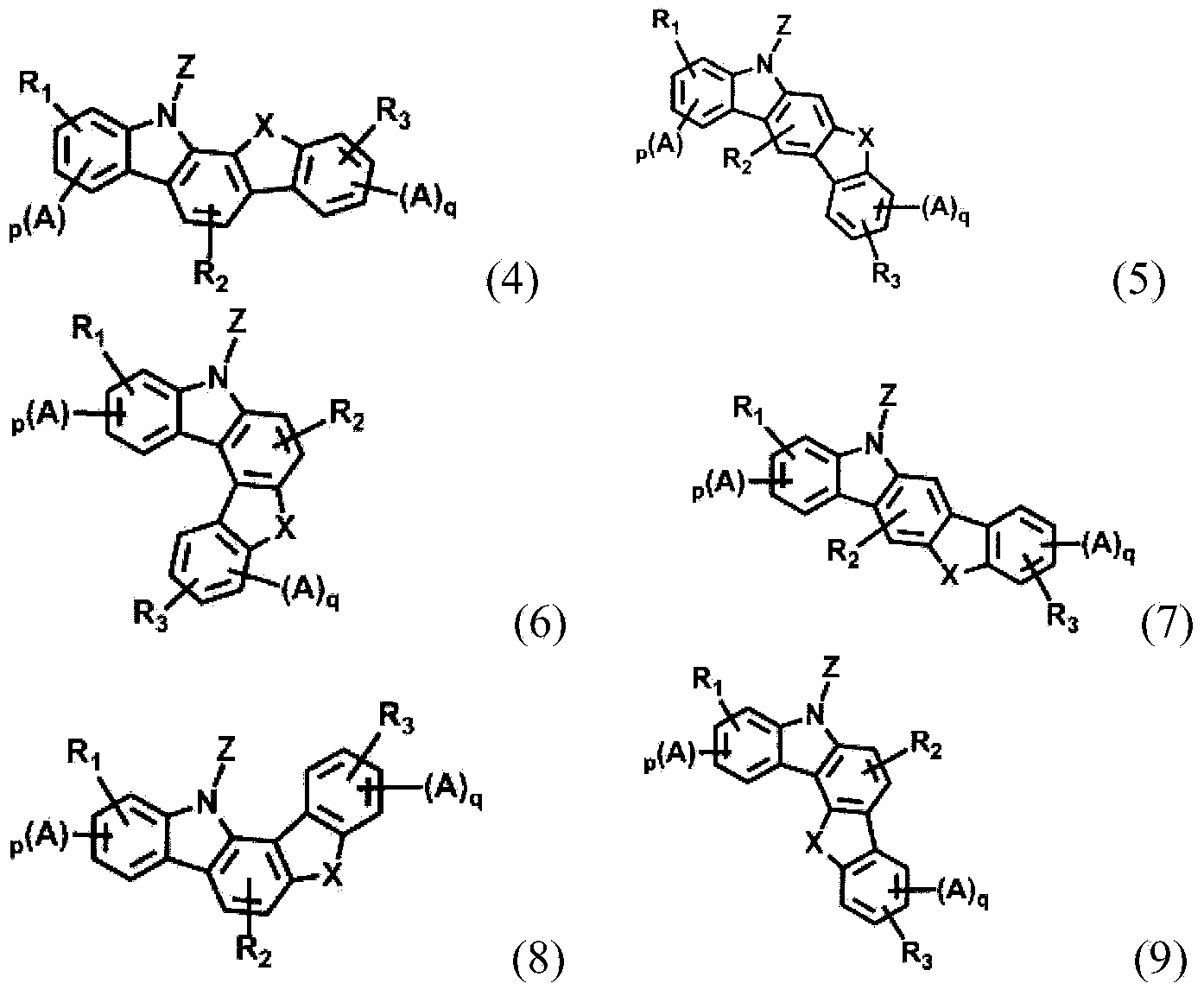
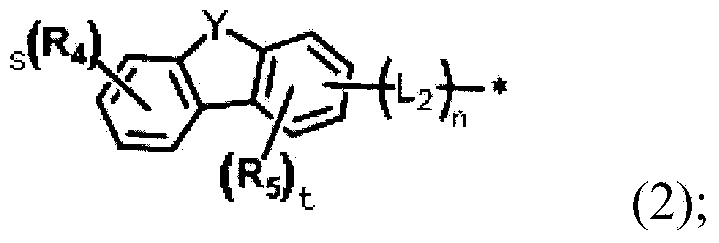Novel organic electroluminescent compounds and organic electroluminescent device comprising the same
A compound and luminescent technology, applied in electroluminescence light sources, organic chemistry, electric solid-state devices, etc., can solve the problems of unsatisfactory working life and luminous efficiency, low glass transition temperature, and degradation of organic EL devices , to achieve the effect of excellent hole transport ability, high luminous efficiency and long driving life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0103] Embodiment 1: the preparation of compound C-1
[0104]
[0105] Preparation of compound C-1-1
[0106] 2-Bromo-7-iodo-9,9-dimethyl-9H-fluorene (25.0 g, 62.6 mmol), 9-phenyl-9H-carbazol-3-ylboronic acid (16.3 g, 56.9 mmol ), tetrakis(triphenylphosphine)palladium(O)[Pd(PPh 3 ) 4 ] (3.6g, 3.1mmol) and Na 2 CO 3 (19.9g, 216.0 mmol) was added to the flask and after dissolving the reaction mixture by adding toluene (400.0ml), ethanol (EtOH) (100.0ml) and distilled water (100.0ml), the reaction mixture was heated at 120°C Stir for 3 hours. After the reaction, the reaction was terminated by slowly adding distilled water, and the organic layer was extracted with vinyl acetate (EA). with MgSO 4 The obtained organic layer was dried to remove residual moisture, and separated by column chromatography to obtain compound C-1-1 (27.5 g, 53.5 mmol, yield: 84%).
[0107] Preparation of compound C-1-2
[0108] Add compound C-1-1 (27.5g, 53.5mmol), 2-chloroaniline (11.2ml,...
Embodiment 2
[0113] Embodiment 2: the preparation of compound C-22
[0114]
[0115] Preparation of Compound C-22-1
[0116] 1,3-Dibromobenzene and sulfuric acid (250.0 ml) were added to the flask and the reaction mixture was cooled to an internal temperature of 0°C. Nitric acid (28.6ml) was slowly added to the flask and the reaction mixture was stirred for 30 minutes. After the reaction was completed, ice water was added to the reaction mixture, and the obtained solid was filtered and rinsed with water. The solid was rinsed with NaOH to make a neutral solid. The solid was separated by column chromatography to obtain compound C-22-1 (60.0 g, 213.5 mmol, yield: 50%).
[0117] Preparation of compound C-22-2
[0118] Compound C-22-1 (60.0g, 213.5 mmol), dibenzo[b, d]thiophen-4-ylboronic acid (40.6g, 177.9 mmol), Pd(PPh 3 ) 4 (8.2g, 7.1mmol) and Na 2 CO 3 (56.6 g, 534.0 mmol) was added to the flask and after dissolving the reaction mixture by adding toluene (520.0 ml), EtOH (2...
Embodiment 3
[0125] Embodiment 3: the preparation of compound C-26
[0126]
[0127] Preparation of compound C-26-1
[0128] Compound C-22-1 (60.0g, 213.5 mmol), dibenzo[b, d]furan-4-ylboronic acid (37.7g, 177.9 mmol), Pd(PPh 3 ) 4 (10.2g, 8.8mmol) and Na 2 CO 3 (56.6 g, 534.0 mmol) was added to the flask and after dissolving the reaction mixture by adding toluene (520.0 ml), EtOH (260.0 ml) and distilled water (260.0 ml), the reaction mixture was stirred at 120° C. for 3 hours . After the reaction, the reaction was completed by slowly adding distilled water, and the organic layer was extracted with EA. with MgSO 4 The obtained organic layer was dried to remove residual moisture, and separated by column chromatography to obtain compound C-26-1 (42.0 g, 114.0 mmol, yield: 54%).
[0129] Preparation of compound C-26-2
[0130] Compound C-26-1 (10.0g, 29.7mmol), 9-phenyl-9H-carbazol-3-ylboronic acid (10.2g, 35.7mmol), Pd(PPh 3 ) 4 (1.4g, 1.2mmol) and K 2 CO 3 (12.3 g, 89....
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com