Environment-sensitive tumor-targeting polymer micelle and preparation method thereof
A polymer gel and tumor-targeting technology, which is applied in the preparation and preparation of tumor-targeted polymer micelles drug delivery system, and in the field of tumor-targeted polymer micelles, can solve the problems of complex peptide synthesis and achieve in vivo safety High, reduce drug leakage, good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
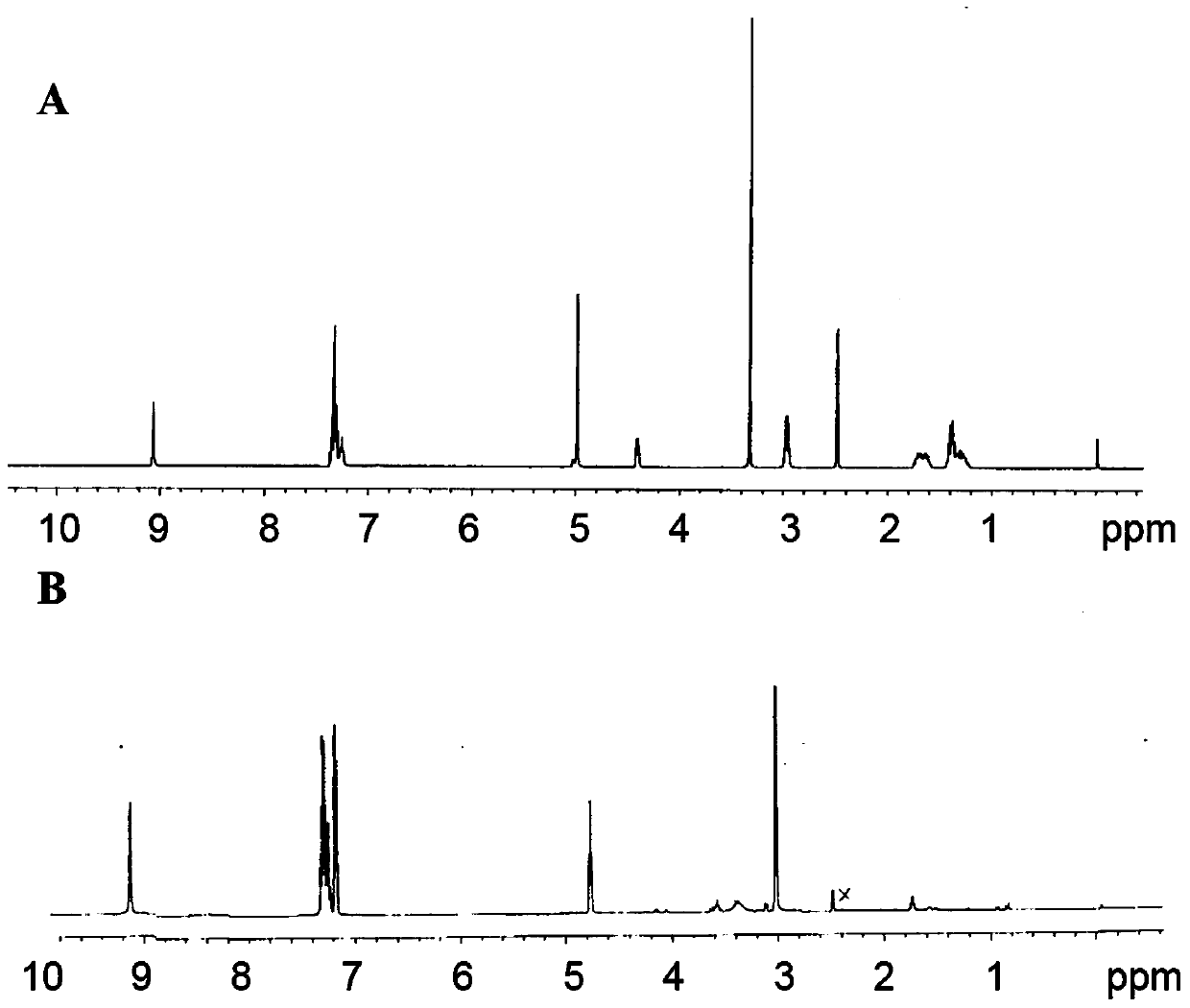
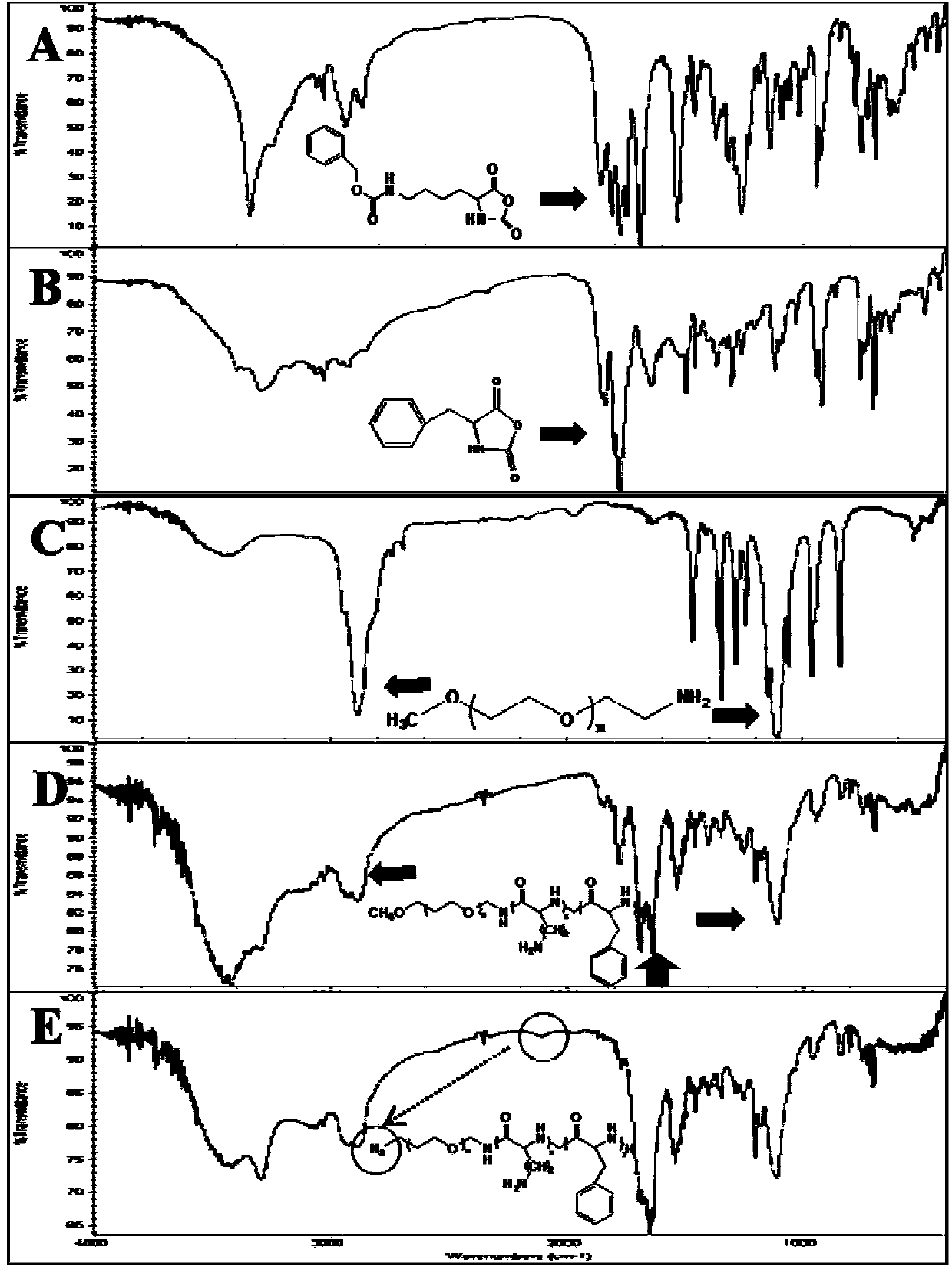
[0065] N 6 -Benzyloxycarbonyl-L-lysine (N 6 -Lys(Z)) and L-phenylalanine (L-Phe) NCA were carried out according to the Fuchs-Farthing method, according to synthetic route 1: Weigh N 6 -Lys(Z) 30g (107mmol), suspended in dry THF (300ml), under nitrogen protection, equilibrated in an oil bath at 50°C for 10min, added 10.58g (35.68mmol) of triphosgene solid, stirred at 50°C for 3h until solution To clarify, the reaction solution was poured into anhydrous n-hexane, and a white precipitate was precipitated, which was filtered and dried to obtain N 6 -Lys(Z)-NCA (compound a), recrystallized from anhydrous THF / n-hexane system to obtain white crystals with a yield of 98%. Similarly, weigh 5g of L-Phe (30.3mmol), suspend it in 50ml of anhydrous THF, operate as above, add 3g of triphosgene solid (12.1mmol), react for 3h, other operations are as above, use anhydrous THF / n-hexane system to weigh Crystallization afforded a white solid, Phe-NCA (compound b), in 96% yield. use 1 H NMR (...
Embodiment 2
[0069] synthetic N 3 - PEG-pLys-pPhe or MPEG-pLys-pPhe polymers, respectively, with N 3 -PEG-NH 2 (MW5000) and MPEG-NH 2 (MW5000) as the initiator, according to the synthetic route 2: weigh CH 3 O-PEG-NH 2 or N 3 -PEG-NH 2 (1g, 0.2mmol) was dissolved in anhydrous DMF (10ml), stirred constantly, weighed N 6 -Lys(Z)-NCA (compound a) 736mg (2.4mmol), was added to the PEG solution under nitrogen protection, and reacted at 50°C for 48h. Weigh 917mg (4.8mmol) of Phe-NCA (compound b), add it into the reaction solution, and react at 50°C for 48h. After the reaction, the reaction solution was poured into anhydrous ether, and a white precipitate was precipitated, which was filtered and dried to obtain a white solid with a yield of 92%. The white solid was added to TFA (10ml)-HBr / HOAc (0.5ml) and reacted for 3h to remove N 6 - benzyloxycarbonyl protecting group (Z). The product was transferred to a dialysis bag (MW1000), dialyzed in 1 L of pure water for 24 hours, and the water...
Embodiment 3
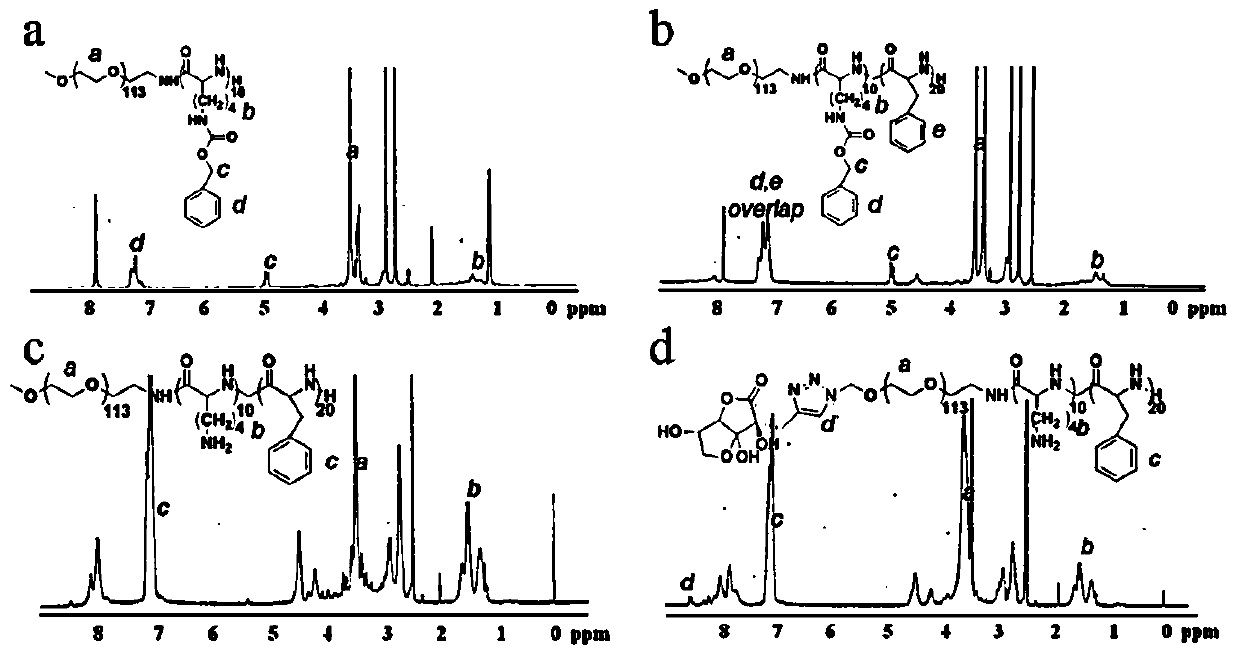
[0073] The propargylated DHA and N 3 -PEG-pLys-pPhe connection, according to synthetic route 3: Weigh polymer N 3 - 500 mg (0.1 mmol) of PEG-pLys-pPhe was dissolved in DMF, under nitrogen protection, and 43 mg (0.2 mmol, 2 eq.) of propargylated DHA was weighed and added to the polymer solution. CuI (0.5eq.) and DIPEA (1eq.) were freshly prepared, added to the reaction solution, and reacted at 30°C for 12h. The reaction solution was transferred to a dialysis bag (MW1000), dialyzed in 1L10mM EDTA-2Na pH7.0 for 24h, transferred to 1L pure water for dialysis for 24h, and freeze-dried to obtain purified DHA-PEG-pLys-pPhe; To characterize (such as figure 2 d).
[0074] Synthetic route 3:
[0075]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com