Preparation method of ferric phosphate
A technology of iron phosphate and phosphoric acid, applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry, etc., can solve the problems of difficult filtration, low capacity, and harm the quality of iron phosphate, and achieve the effect of stable iron content and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
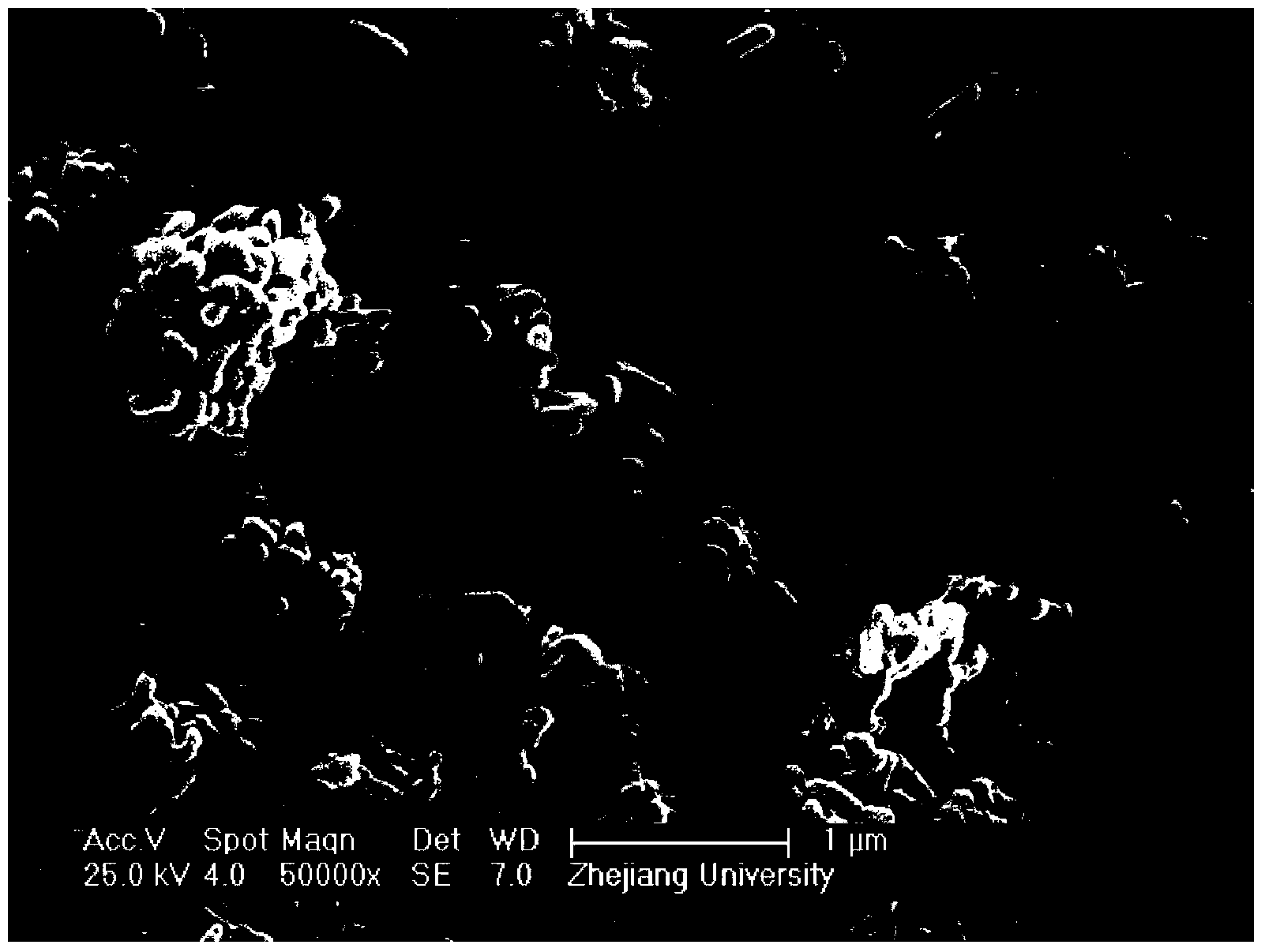
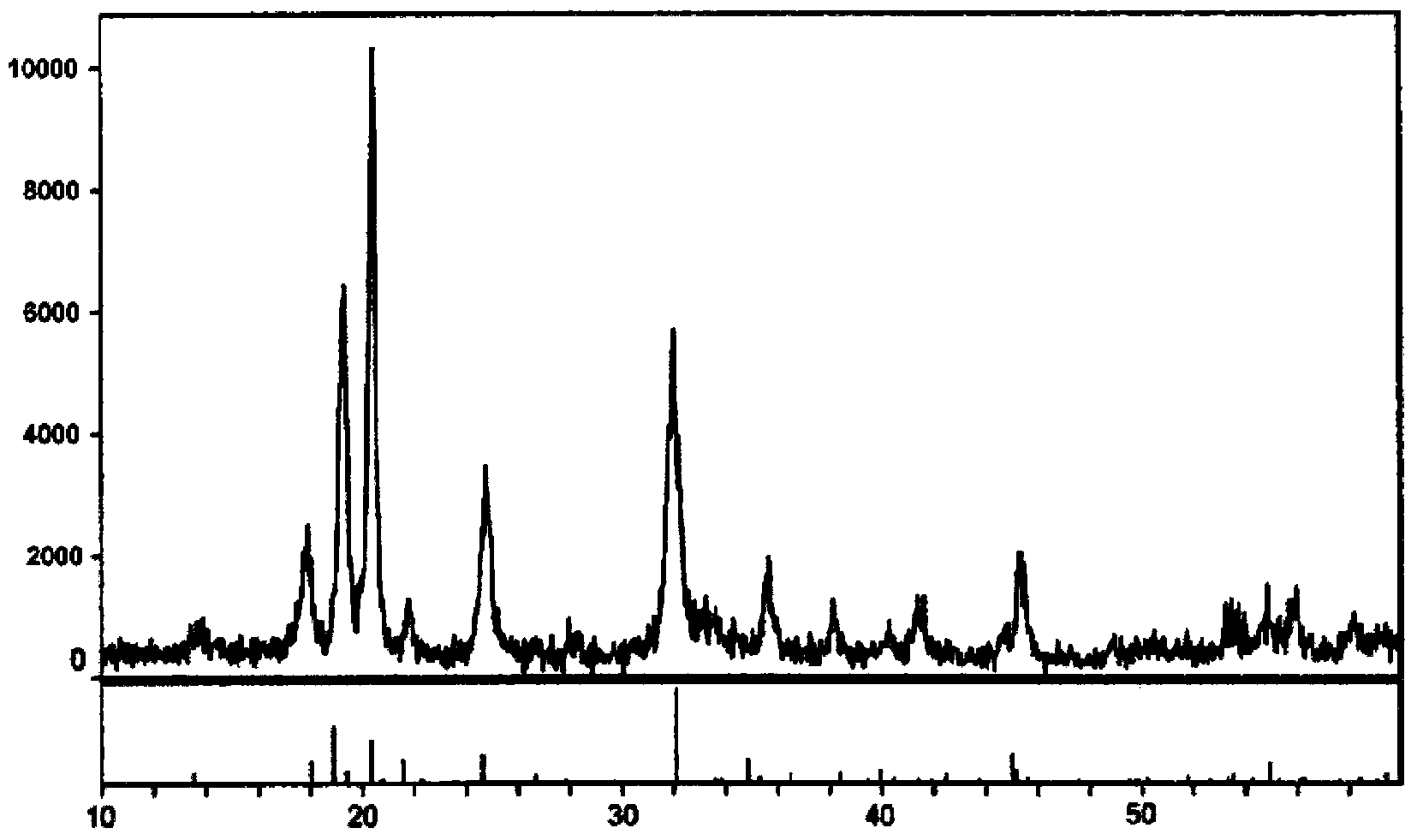
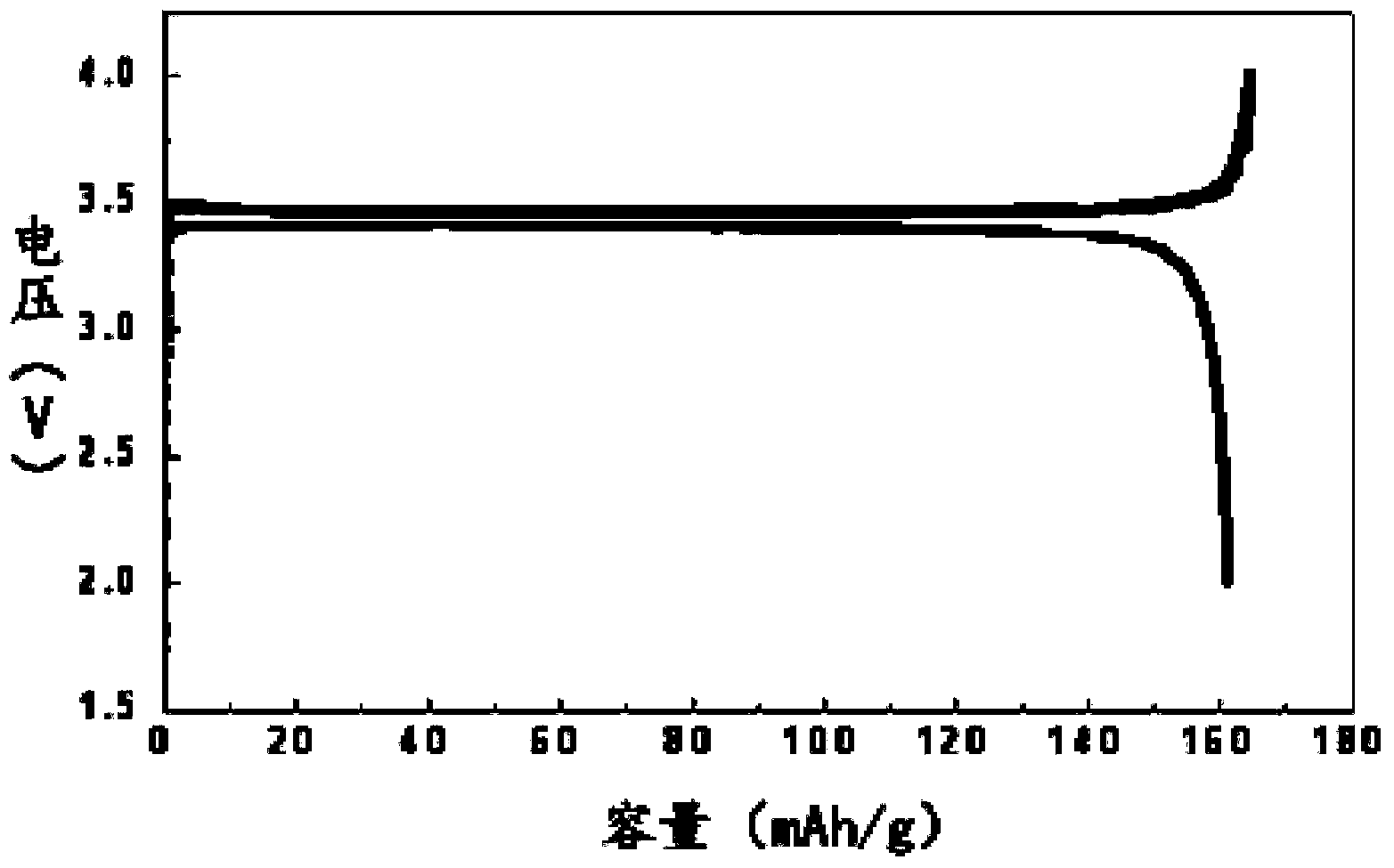
Image
Examples
Embodiment 1
[0025] Dissolve ferrous sulfate (280.8g, 99-101%) in 1L of deionized water, add 6g of sulfuric acid, stir evenly, and filter the solution to remove insoluble matter.
[0026] Diammonium hydrogen phosphate (133.4 g, 99%) was dissolved in 0.5 L of deionized water, stirred evenly, and the solution was filtered to remove insoluble matter.
[0027] The above two solutions were mixed under rapid stirring, and the solution did not precipitate.
[0028] Hydrogen peroxide (78 g, 30%) was slowly added to the above mixture, the pH increased during the oxidation process, and a suspension of yellow-white precipitate was obtained.
[0029] Slowly raise the temperature of the solution to 80° C., keep it warm for 2.5 hours, wash and filter to obtain a light powdery white filter cake.
[0030] The filter cake was sintered in a muffle furnace at 350°C for 6 hours to obtain 140.2 grams of dry powder, which was ferric phosphate.
[0031] Material analysis is: Fe / P molar ratio 0.995, sulfur cont...
Embodiment 2
[0039] Dissolve ferrous sulfate (224.6g, 99-101%) in 1L of deionized water, stir evenly, and filter the solution to remove insoluble matter.
[0040] Dissolve trisodium phosphate (132.5 g, 99%) in 1 L of deionized water, stir evenly, and filter the solution to remove insoluble matter.
[0041] The above two solutions were mixed under rapid stirring; a blue ferrous phosphate precipitate was obtained.
[0042] Take hydrogen peroxide (92g, 30%) and slowly add the mixed solution.
[0043] Slowly add hydrogen peroxide to the ferrous phosphate suspension, and continue to stir rapidly to gradually oxidize to obtain pure ferric phosphate precipitation.
[0044] Add 12 g of sulfuric acid to this suspension and mix well.
[0045]After stirring slowly for 60 minutes, slowly raise the solution temperature to 85°, keep the temperature for 2.5 hours, filter and wash to obtain light pink dihydrate crystalline ferric phosphate.
[0046] The filter cake was sintered in a muffle furnace at 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com