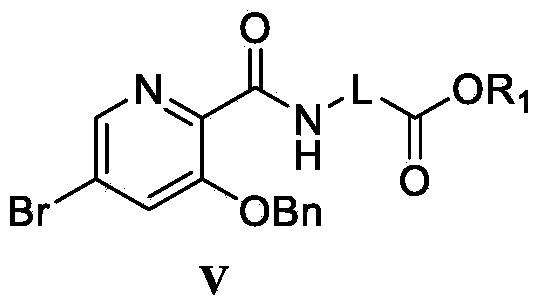
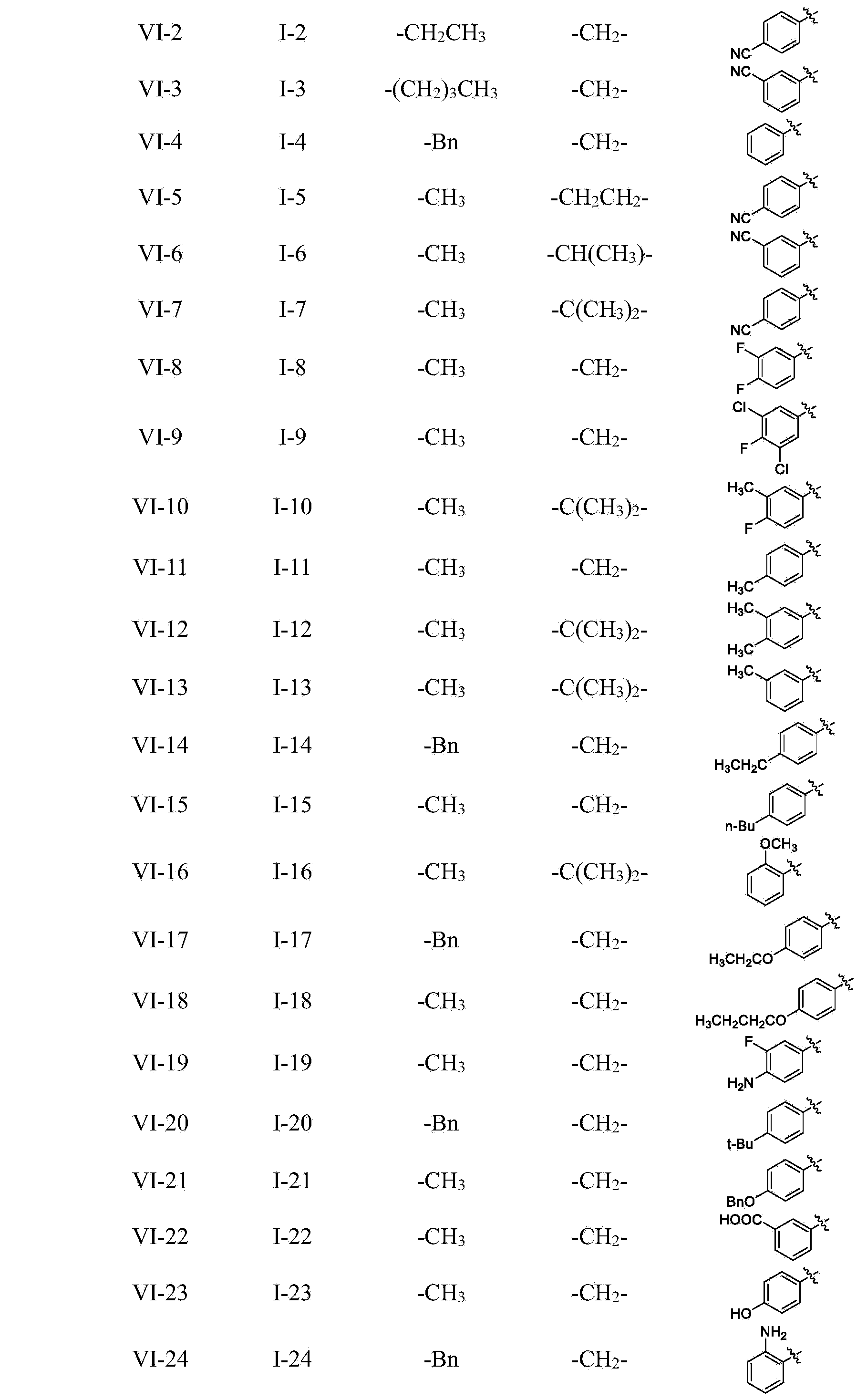
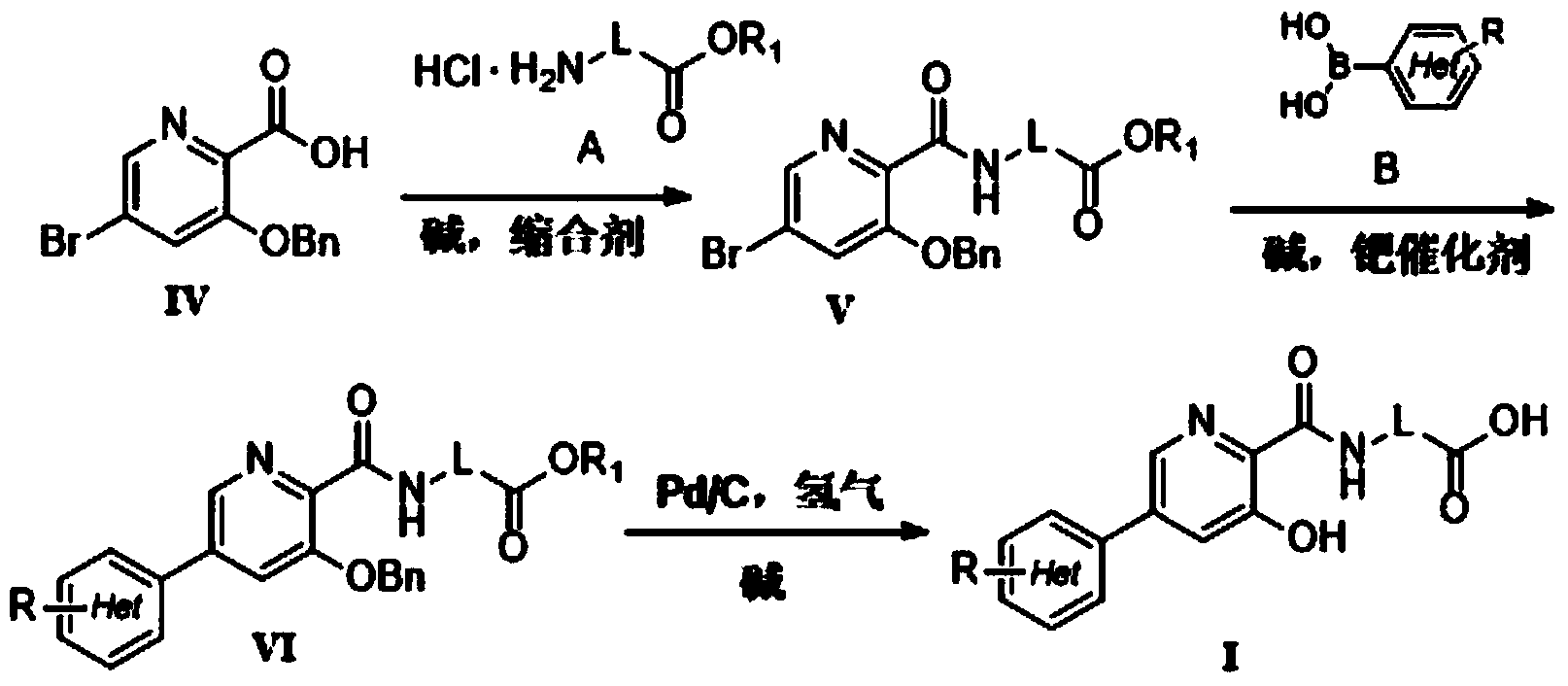
Preparation method and intermediate of 3-hydroxyl-5-aryl pyridine-2-formamide derivative
A technology of dimethylformamide and alkyl, which is applied in the field of medicinal chemistry, can solve the problems of unfavorable scale-up production, long synthetic route, cumbersome treatment, etc., and achieve the effects of high total yield, low cost, and shortened reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Preparation of 2-[3-benzyloxy-5-(4-fluorophenyl)pyridine-2-carboxamido]acetic acid (I-1)
[0051] (1) Preparation of 2-(3-benzyloxy-5-bromopyridine-2-carboxamido)methyl acetate (V-1)
[0052] Intermediate IV (49.3g, 0.16mol) was dissolved in 500mL of dichloromethane, then 60ml of trimethylamine and 1-hydroxybenzotriazole (32.6g, 0.24mol) were added, and after stirring for 10min, 1-ethyl-( 3-Dimethylaminopropyl) carbodiimide hydrochloride (45.9g, 0.24mol), glycine methyl ester hydrochloride (24g, 0.19mmol). Reaction at room temperature for 10h. , after the reaction, washed with saturated sodium bicarbonate (500mL), water (2×500mL) and saturated brine (500mL) respectively. The organic phase was dried over anhydrous sodium sulfate, and after vacuum distillation, the crude product was recrystallized with 95% ethanol to obtain 8.2 g of white solid compound V-15. Yield 96%. m.p.101.2-102.9℃. 1 H-NMR (300MHz, CDCl 3 )δ3.79(s,3H,-OC H 3 ),4.27(d,2H,-NH CH 2 -),5.26(s,...
Embodiment 2
[0058] Preparation of 2-[3-benzyloxy-5-(4-cyanophenyl)pyridine-2-carboxamido]acetic acid (I-2)
[0059] (1) Preparation of 3-benzyloxy-5-bromo-2-cyanopyridine (III)
[0060] Benzyl alcohol (59.08 mL, 0.57 mol) and 60% NaH (2.60 g) were thrown into 1 L of anhydrous THF under ice-cooling conditions, stirred until no bubbles were generated, and reacted at room temperature for 2 h. The reaction solution was added dropwise to a solution of 5-bromo-2-cyano-3-nitropyridine II (100 g, 0.46 mol) in anhydrous THF (0.5 L). After the addition was complete, the reaction was maintained at room temperature for 24 h. After the reaction was completed, water (200 mL) was added to quench the reaction, and the organic solvent in the reaction solution was distilled off under reduced pressure, then dichloromethane (1 L) was added, and water (3×500 mL) and saturated brine (500 mL) were washed successively respectively, Keep the organic phase, dry it over anhydrous sodium sulfate, and distill under ...
Embodiment 3
[0070] Preparation of 2-[3-benzyloxy-5-(3-cyanophenyl)pyridine-2-carboxamido]acetic acid (I-3)
[0071] (1) Preparation of 2-(3-benzyloxy-5-bromopyridine-2-carboxamido)butyl acetate (V-3)
[0072] Intermediate IV (49.3g, 0.16mol) was dissolved in 500mL of toluene, and then 73g of p-dimethylaminopyridine and benzotriazol-1-yloxytris(dimethylamino)phosphonium hexafluorophosphate were added (0.24mol), after stirring for 10min, add 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride (45.9g, 0.24mol), butyric acid butyl ester hydrochloride (31.98g , 0.19 mol). Reaction at 50°C for 6h. Post-treatment was the same as step (1) of Example 1 to obtain 77 g of white solid compound V-3. Yield 96%. m.p.105.4-107.8℃. 1 H-NMR (300MHz, CDCl 3 )δ0.9(t,3H,-CH 2 CH 3 ),1.45(m,2H,- CH 2 CH 3 ),1.62(m,2H,- CH 2 CH 2 -),4.15(m,2H,-O CH 2 - ),4.25(d,2H,-NH CH 2 -),5.26(s,2H,-Ph CH 2 ),7.28-7.44(m,3H,Ar- H ),7.51-7.53(m,2H,Ar- H ),7.57(d,1H,Py- H ),8.311(s,1H,-N H CO-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com