Process for synthesizing high-content dehydronandrolon acetate
A technique for the synthesis of dehydronandrolone acetate, applied in the direction of steroids, organic chemistry, etc., to achieve the effects of simple synthesis process conditions, improved economic benefits, and high content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
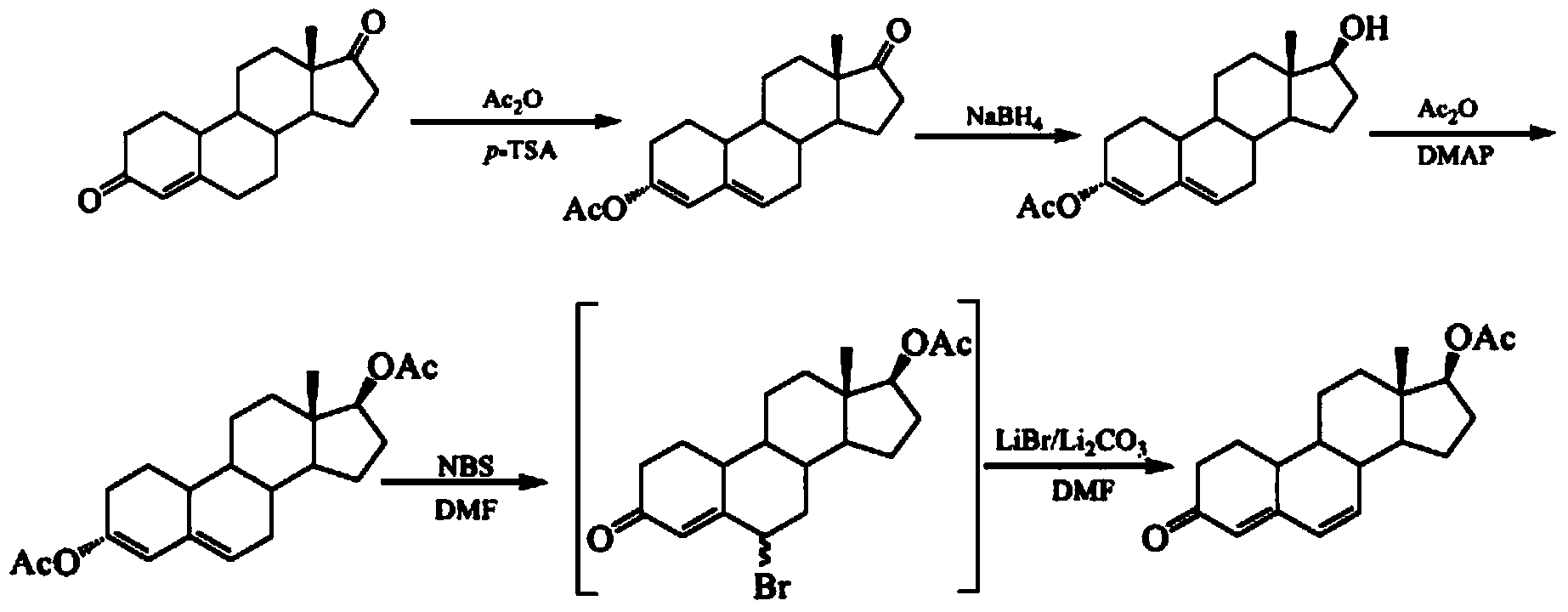
Examples
Embodiment 1
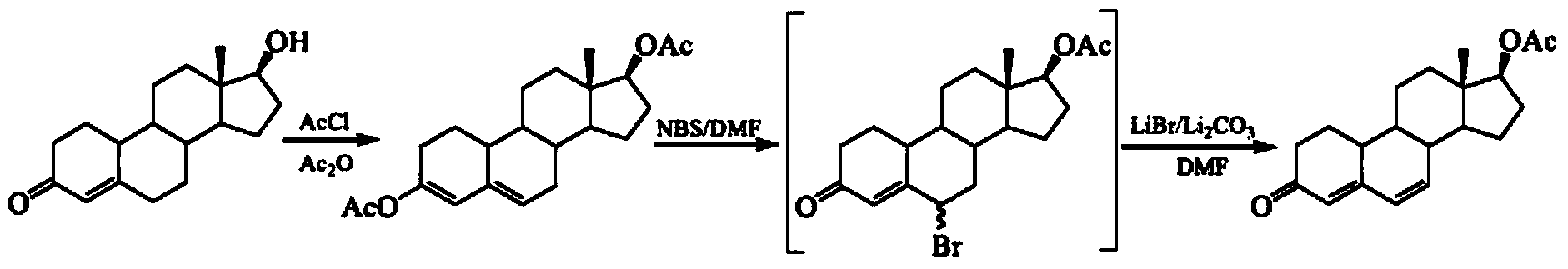
[0056] Step 1: Compound 2——Δ 3,5 - the preparation method of estro-3-acetoxy-17-one (2):
[0057] Take 1.0g (3.67mmol) of estro-4-ene-3,17-dione (1), add 5.0ml of acetic anhydride to make it just dissolve, then add 0.05g (0.17mmol) of p-toluenesulfonic acid, at 15°C After reacting for about 15 minutes, the reaction solution became turbid, and white solids precipitated out. After continuing the reaction for 2 hours, add 80ml of saturated sodium bicarbonate solution and stir for 1 hour. When the solids were dispersed in the solution in powder form, filter directly, wash the solids twice with water, and dry them in the air. Solid, to obtain off-white powdery solid Δ 3,5 -Estro-3-acetoxy-17-one (2) 1.12g, yield 97.2%, mp157-159°C;
[0058] Step 2: Compound 3—the preparation method of estra-3,5-diene-17β-alcohol-3-acetate (3):
[0059] Put 1.0g (3.18mmol) of compound 2 in the reaction vessel, add 30ml of methanol, but it cannot be dissolved completely, add 0.12g (0.32mmol) of po...
Embodiment 2
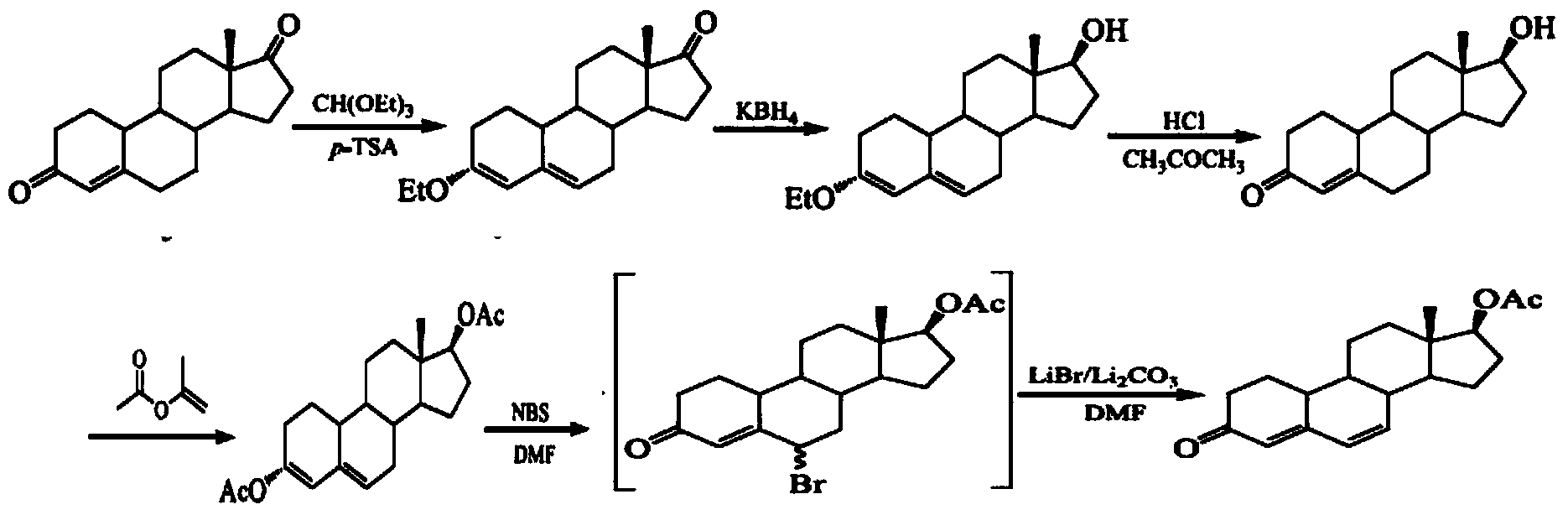
[0067] Step 1: Compound 2——Δ 3,5 - the preparation method of estro-3-acetoxy-17-one (2):
[0068] Take 1.0g (3.67mmol) estro-4-ene-3,17-dione (1), add 9.0ml isopropenyl acetate to make it just dissolve, then add 0.05g (0.17mmol) p-toluenesulfonic acid, dehydrate After reacting for 15 minutes, the reaction solution became turbid. Continue to react at room temperature for 1 hour, then add 80ml of saturated sodium bicarbonate solution and stir for 1 hour. When the solid is dispersed in the solution in powder form, filter directly, wash the solid with water twice, and dry the solid to obtain an off-white powder. solid Δ 3,5 -Estro-3-acetoxy-17-one (2) 1.12g, yield 97.2%, mp157-159°C;
[0069] Step 2: Compound 3—the preparation method of estra-3,5-diene-17β-alcohol-3-acetate (3):
[0070] Put 1.0g (3.18mmol) of compound 2 in the reaction vessel, add 30ml of methanol (not completely dissolved), and add 0.12g (0.32mmol) of sodium borohydride at 15°C, after 30min the reaction solut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com