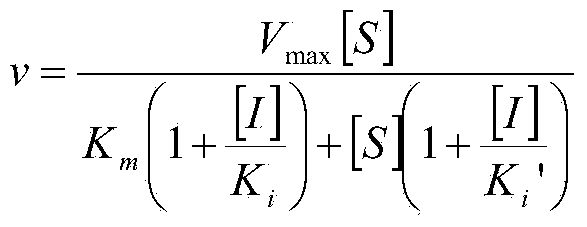Cyclodextrin glucosyltransferase mutant weakened via product inhibition
A glucose-based and mutant technology, applied in the fields of genetic engineering and enzyme engineering, can solve the problems of low starch conversion rate, decreased CGT enzyme cyclization activity, and limited application of cyclodextrin, and achieves benefits for industrial production and increased yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 Determination of Mutation Sites
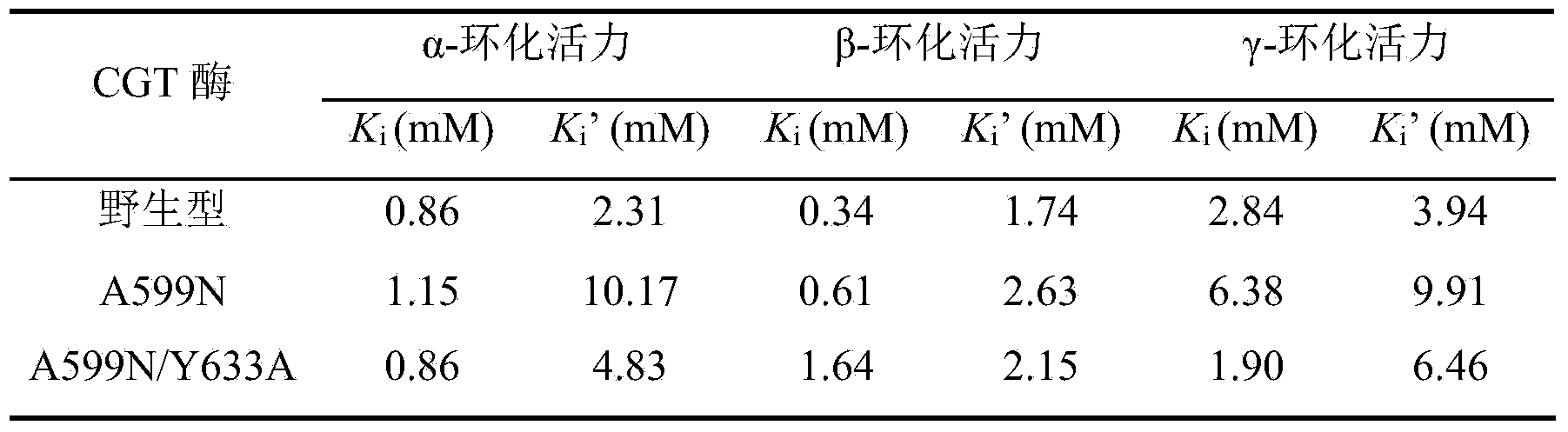
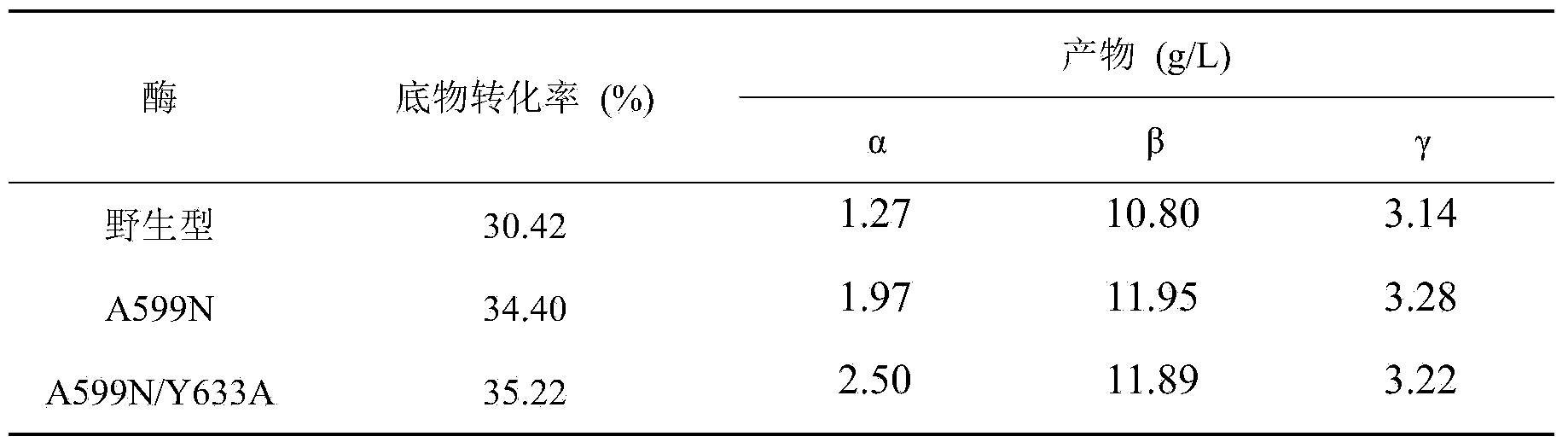
[0025] There are three maltosyl-binding sites in CGTase, which are maltosyl-binding site 1 (abbreviated as MBS1), maltosyl-binding site 2 (abbreviated as MBS2) and maltosyl-binding site 3 (MBS3). During the reaction between starch and CGTase to generate cyclodextrin, the cyclodextrin product will combine with MBS2 in CGTase, which prevents starch from entering the active center and inhibits the cyclization activity of CGTase, resulting in a low yield of cyclodextrin. MBS2, derived from the CGTase of Bacillus circulans STB01, is composed of 39 amino acid residues from positions 598 to 636, of which Ala599 and Tyr633 can interact with cyclodextrin through hydrogen bonds, which may be related to It is related to hindering the entry of starch into the active center. Therefore, if the spatial structure of these two sites is changed, it may be possible to weaken the inhibition of cyclodextrin on CGTase activity, so that CGTase can ...
Embodiment 2
[0026] The preparation of embodiment 2 mutant A599N, A599N / Y633A
[0027] (1) Site-directed mutation
[0028] Site-directed mutagenesis of the single mutant A599N: using rapid PCR technology, the expression vector pST / cgt (or other common Bacillus subtilis expression vectors, such as pUB110, pC194, etc.) containing the wild CGTase gene was used as a template for site-directed mutation.
[0029] Primers for site-directed mutagenesis introducing the Asn599 codon:
[0030] Forward primer: 5'-AACGCGACGACG AAT CTTGGGCA-3', the underline is the mutant base,
[0031] Reverse primer: 5'-TGCCCAAG ATT CGTCGTCGCGTT-3', the underline is the mutant base;
[0032] Site-directed mutation of the double mutant A599N / Y633A: using rapid PCR technology, the site-directed mutation was performed using the expression vector pST / cgt of the single mutant A599N gene as a template.
[0033] Primers for site-directed mutagenesis introducing the Ala633 codon:
[0034] Forward primer: 5'-GTCGTTTACCA...
Embodiment 3
[0041] Embodiment 3 Enzyme assay analysis
[0042] (1) Determination of enzyme activity
[0043] Determination of α-cyclization activity: Take 0.1 mL of appropriately diluted enzyme solution, add 0.9 mL of 1% (w / v) maltodextrin (DE=5) solution prepared in advance with 50 mM phosphate buffer (pH 6.0) In a test tube, react at 50°C for 10min, add 1.0mL of 1.0N hydrochloric acid to stop the reaction, then add 1.0mL of 0.1mM methyl orange solution prepared with 50mM phosphate buffer, keep warm at 20°C for 15min, and measure the absorbance at 505nm . Using the inactivated enzyme as a blank, the content of α-cyclodextrin was determined corresponding to the α-cyclodextrin standard curve. One enzyme activity unit is defined as the amount of enzyme required to generate 1 μmol of cyclodextrin per minute under the above conditions.
[0044] Determination of β-cyclization activity: Take 0.1 mL of appropriately diluted enzyme solution, add 0.9 mL of 1% (w / v) maltodextrin (DE=5) solution ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com