Fullerene poly(glycidyl nitrate), preparation method and application thereof
A technology of polyglycidyl ether and nitrate ester, which is applied in the field of energetic combustion catalysts and its preparation, can solve problems such as limiting the scope of application, and achieve the effects of simple and convenient operation, high yield, and reduced pressure index
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
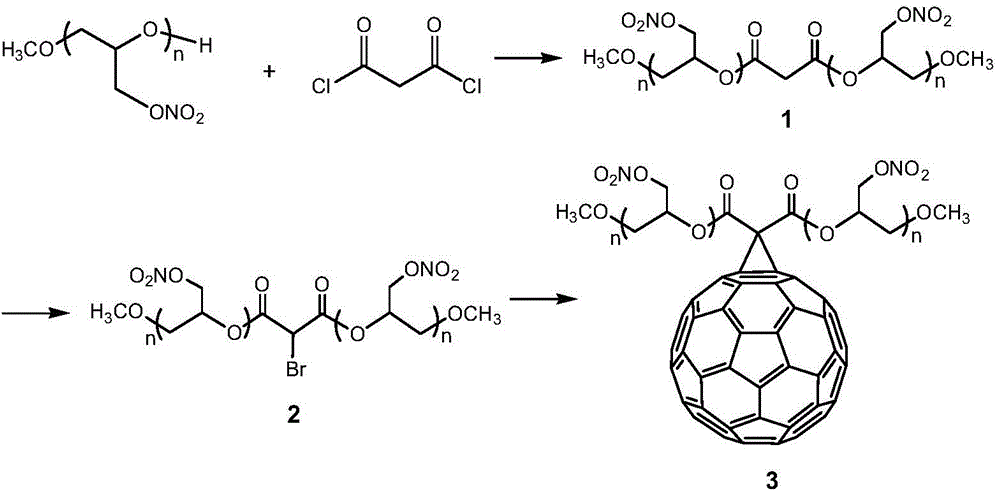
[0035] Embodiment 1: Preparation of fullerene polyglycidyl ether nitrate 3
[0036] (1) 5.54g of monomethyl polyglycidyl ether nitrate, 0.65mL of acid-binding agent triethylamine and 45mL of methylene chloride were put into the reactor, and malonyl chloride (0.59g) was mixed with 50mL of dichloromethane at 0°C. Diluted methyl chloride was slowly added to the solution and reacted at room temperature for 11 h. The reaction solution was washed with water to a pH of about 7.0, dried and then evaporated to remove the solvent to obtain 4.5 g of malonate dimonomethyl polyglycidyl ether nitrate 1 .
[0037] (2) Add 4.5g of the above-mentioned dimethyl malonate dimethyl polyglycidyl ether nitrate 1 and 100mL of methylene chloride in the reaction flask, and drop 1.5g of bromine into the reaction solution dropwise at room temperature until the reaction solution No fading, and then continue to stir the reaction for 6h. After the reaction was completed, it was washed three times with sat...
Embodiment 2
[0044] Embodiment 2: Preparation of fullerene polyglycidyl ether nitrate 3
[0045] (1) Put 2.26g of monomethyl polyglycidyl ether nitrate, 0.56mL of acid-binding agent pyridine and 20mL of dichloromethane into the reactor, add malonyl chloride (0.59g) with 50mL of dichloromethane After dilution, it was slowly dropped into the solution and reacted at room temperature for 11 hours. The reaction solution was washed with water to a pH of about 7.0, dried and then evaporated to remove the solvent to obtain 2.3 g of malonate dimonomethyl polyglycidyl ether nitrate.
[0046] (2) Add 2.3g of the above-mentioned dimethyl polyglycidyl malonate nitrate 1 and 50mL of dichloromethane into the reaction flask, and add 2.0g of N-bromosuccinimide to the reaction solution at room temperature , and then stirred at 60°C for 4h. After the reaction, wash with saturated sodium bromide solution and distilled water three times in sequence, dry with anhydrous sodium sulfate, filter, and distill the ...
Embodiment 3
[0053] Embodiment 3: Preparation of fullerene polyglycidyl ether nitrate 3
[0054] (1) Under nitrogen or argon atmosphere, put 2.27g monomethyl polyglycidyl ether nitrate, 0.60mL acid-binding agent N, N-dimethylformamide and 25mL methylene chloride into the reactor, 40 Dilute malonyl chloride (0.59g) with 50mL of dichloromethane at °C and slowly drop into the solution, and react at room temperature for 6h. The reaction solution was washed with water to a pH of about 7.0, dried and then evaporated to remove the solvent to obtain 2.5 g of malonate dimonomethyl polyglycidyl ether nitrate 1 .
[0055] (2) Add 2.5g of the above-mentioned dimethyl malonate dimethyl polyglycidyl ether nitrate 1 and 50mL of methylene chloride in the reaction flask, and drop 1.7g of bromine into the reaction solution dropwise at room temperature until the reaction solution No fading, and then continue to stir the reaction for 6h. After the reaction was completed, it was washed three times with satur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com