Method of extracting lithium salt from lepidolite
A lepidolite and lithium extraction technology, which is applied in the field of lithium salt extraction, can solve the problems of increasing the loss rate of rare metal materials, reducing the recycling rate of rare metals, and long heating time at high temperature, so as to reduce equipment damage and environmental impact Pollution, improving extraction utilization, and reducing energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
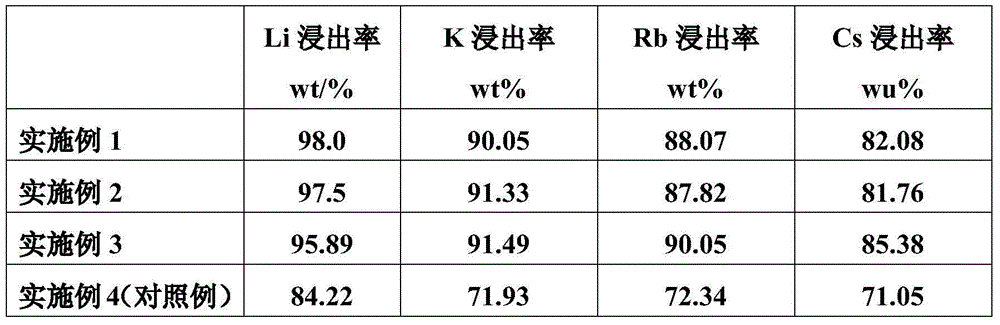
Embodiment 1
[0019] Embodiment 1: The concentration involved in the embodiment is the mass concentration.
[0020] Get its main chemical composition of the lepidolite raw material that Yichun tantalum-niobium-lithium ore produces as follows table 1 (wt%) surplus is fluorine; Table 1 is:
[0021] Li 2 o
K 2 o
Na 2 o
Al 2 o 3
SiO 2
Fe 2 o 3
Rb 2 o
Cs 2 o
4.8
10.56
0.59
24.0
53.57
0.37
1.72
0.30
[0022] 1) Lepidolite with CaO and Ca(CH 3 COO) 2 Roasted at a high temperature by a plasma generator and defluorinated:
[0023] The above-mentioned lepidolite ore was crushed to 200 mesh, and mixed with CaO and Ca(CH 3 COO) 2 Lepidolite ore powder according to mass ratio: CaO: Ca(CH 3 COO) 2 =1:0.1:0.05 for mixed ball milling, mixed for 0.5h, and then placed in a plasma generator for heating and roasting, controlling the roasting temperature to 1500°C, and the roasting time was 30 minutes; at th...
Embodiment 2
[0031] 1) with the lepidolite ore powder described in example 1 and CaO and Ca(CH 3 COO) 2 Lepidolite ore powder according to mass ratio: CaO: Ca(CH 3 COO) 2 =1:0.4:0.01 Mixing ball milling, mixing for 2 hours, and then heating and roasting in a plasma generator, controlling the roasting temperature to 2000°C, and roasting time for 10 minutes, to obtain roasted material; after that, grind the roasted material to obtain roasted powder material;
[0032] The roasting powder and the mass concentration are 15% dilute sulfuric acid solution and (NH 4 ) 2 SO 4 Mix according to the mass ratio of 1:4:0.05, and heat up to 100°C, pressurize and control the reaction pressure at 2.5 atmospheres, stir to make it fully react, control the reaction time for 2 hours, and defluoride again Treatment (further removal of fluorine remaining in the lepidolite raw material) to form a solid-liquid mixed solution containing sulfate;
[0033] Filtrate and separate the solid-liquid mixed solution,...
Embodiment 3
[0038] 1) with the lepidolite ore powder described in example 1 and CaO and Ca(CH 3 COO) 2 Lepidolite ore powder according to mass ratio: CaO: Ca(CH 3 COO) 2 =1:0.3:0.03 Mixing ball milling, mixing for 1 hour, then heating and roasting in a plasma generator, controlling the roasting temperature to 1800°C, and roasting time for 20 minutes, to obtain roasted material; after that, grind the roasted material to obtain roasted powder material;
[0039] The roasting powder and the mass concentration are 25% dilute sulfuric acid solution and (NH 4 ) 2 SO 4 Mix according to the mass ratio of 1:3:0.15, and heat up to 90°C, pressurize and control the reaction pressure at 2 atmospheres, stir to make it fully react, control the reaction time for 3 hours, and perform defluorination again Treatment (further removal of fluorine remaining in the lepidolite raw material) to form a solid-liquid mixed solution containing sulfate;
[0040] Filtrate and separate the solid-liquid mixed solut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com