Synthesis and curing method of carborane benzo oxazine resin
A technology of benzoxazine and carborane, which is applied in the field of benzoxazine prepolymers containing carborane, can solve the problems that the addition reaction cannot be carried out, achieve strong thermal stability and thermal oxygen stability, and Handling of simple, short reaction time effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
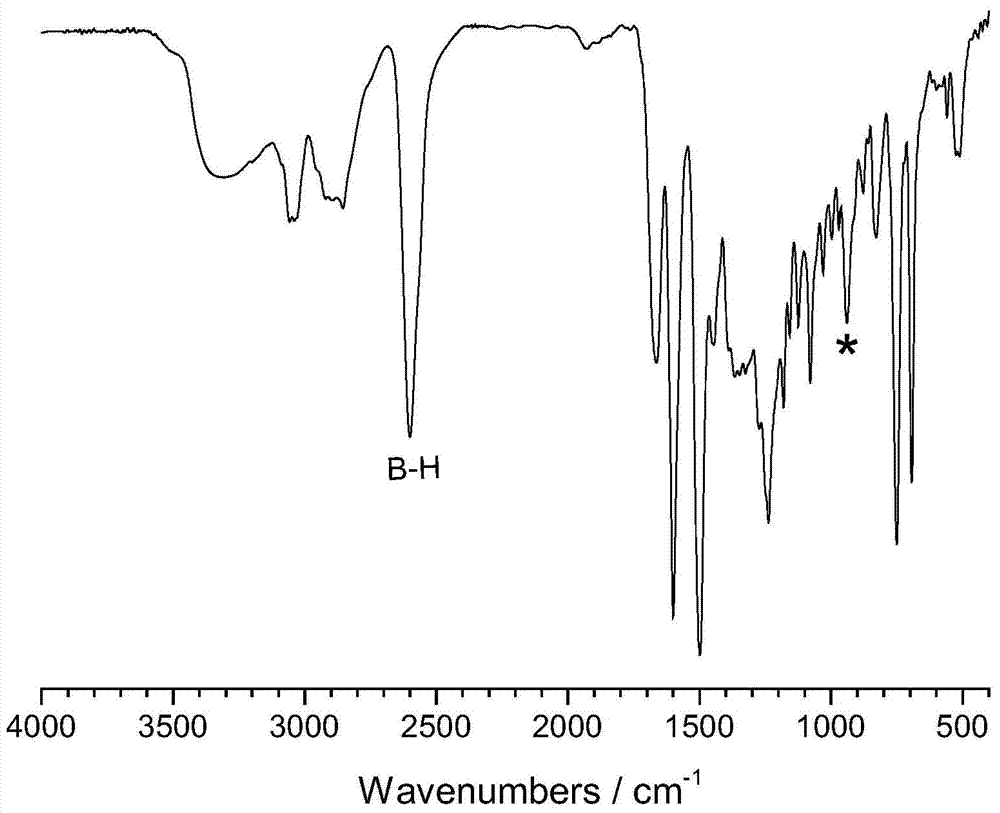
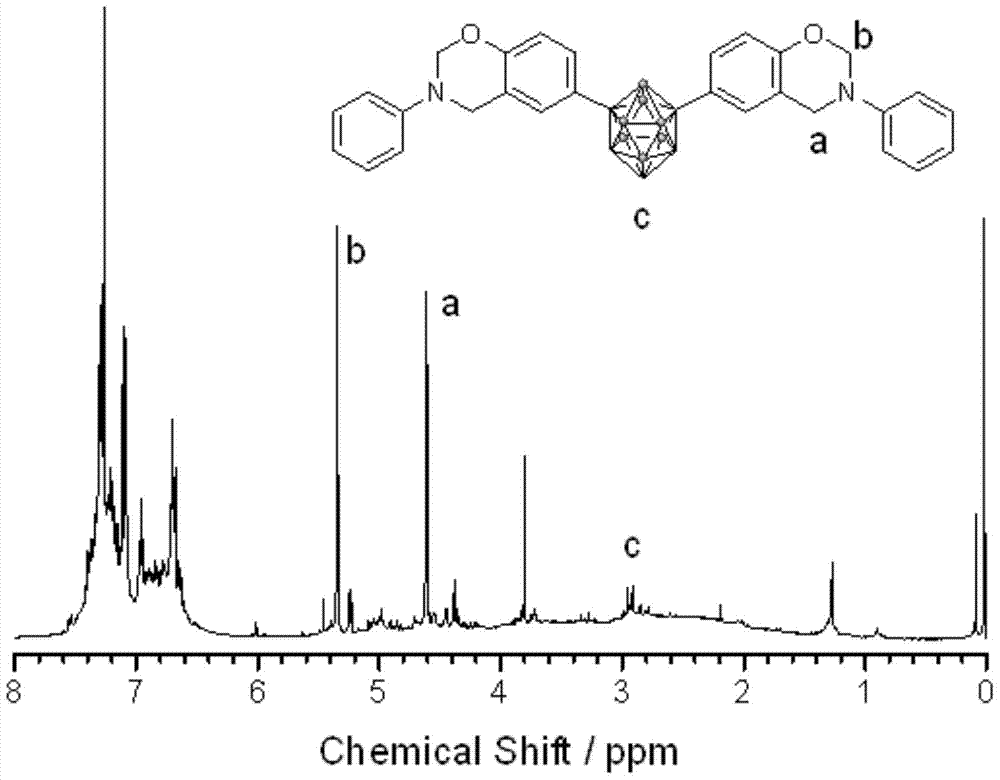
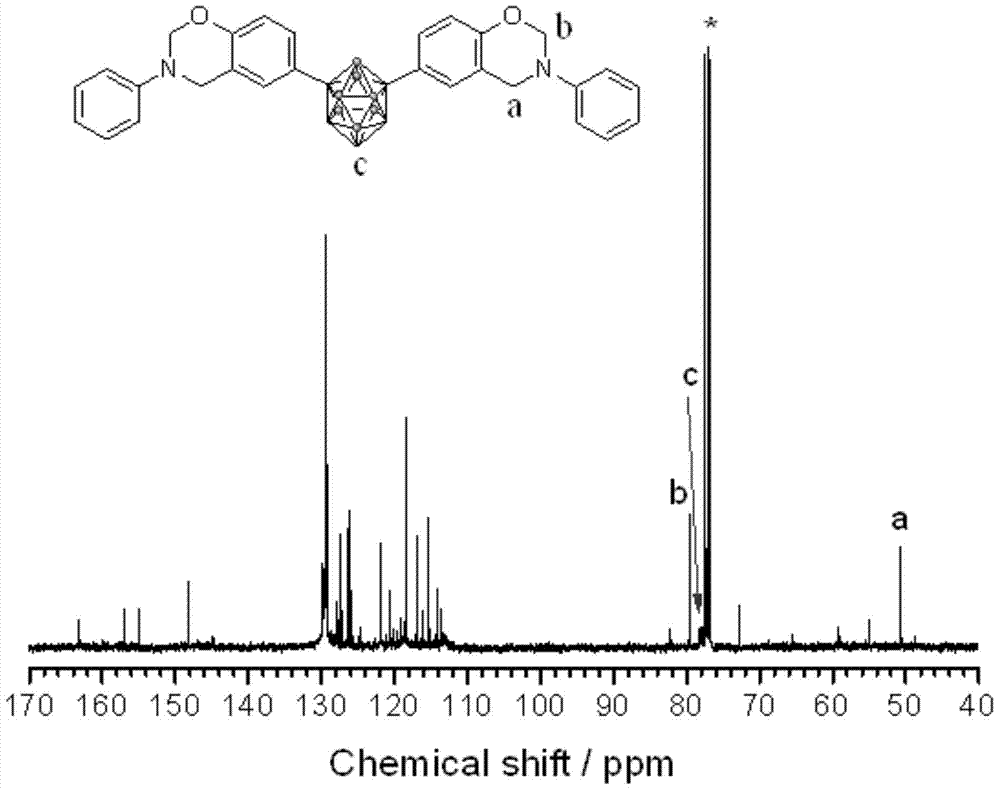
[0036] First, a 100mL three-neck flask equipped with a magnetic stirrer, a thermometer and a reflux condenser was placed in an ice bath, and then a mixed solution of formalin (37%) (0.3294g) and 8mL of dioxane was added to keep the temperature at 10°C Next, a mixed solution of aniline (0.1867 g) and 2 mL of dioxane was subsequently added, and stirred for 30 min. Dissolve 1,7-carborane bisphenol (0.3268g) in 10mL of dioxane, slowly add it dropwise to the above solution, raise the temperature to 94°C, and react for 8h. After the reaction, remove the solvent by rotary evaporation, dissolve the obtained viscous liquid in 80 mL of chloroform, wash with 200 mL of 1.5 mol / L NaOH solution for several times, and finally wash with deionized water until neutral, remove the chloroform by rotary evaporation, and dry in vacuum at 70 °C. Dry for 5h to obtain the product. FTIR,ν(cm -1 , KBr tablet): 939.42 (characteristic peak of oxazine ring), 1498.02 and 1599.45 (skeleton vibration of ben...
Embodiment 2
[0038] First, a 100mL three-neck flask equipped with a magnetic stirrer, a thermometer and a reflux condenser was placed in an ice bath, and then a mixed solution of formalin (37%) (0.3278g) and 8mL of dioxane was added to keep the temperature at 10°C. Next, a mixed solution of methylamine (0.0622 g) and 2 mL of dioxane was subsequently added, and stirred for 30 min. Dissolve 1,2-carborane bisphenol (0.3264g) in 10mL of dioxane, slowly add it dropwise to the above solution, raise the temperature to 100°C, and react for 8h. After the reaction, remove the solvent by rotary evaporation, dissolve the obtained viscous liquid in 80 mL of chloroform, wash with 200 mL of 1.5 mol / L NaOH solution for several times, and finally wash with deionized water until neutral, remove the chloroform by rotary evaporation, and dry in vacuum at 70 ° C. Dry for 5h to obtain the product. FTIR,ν(cm -1 , KBr tablet): 934.20 (characteristic peak of oxazine ring), 751.53 and 837.97 (C-H bending vibratio...
Embodiment 3
[0040] First, a 100mL three-necked flask equipped with a magnetic stirrer, a thermometer and a reflux condenser was placed in an ice bath, then a mixed solution of formalin (37%) (0.3275g) and 8mL of dioxane was added to keep the temperature at 10°C Thereafter, a mixed solution of allylamine (0.1143 g) and 2 mL of dioxane was subsequently added, and stirred for 30 min. Dissolve 1,7-carborane bisphenol (0.3277g) in 10mL of dioxane, slowly add it dropwise to the above solution, raise the temperature to 94°C, and react for 6h. After the reaction, remove the solvent by rotary evaporation, dissolve the obtained viscous liquid in 80 mL of chloroform, wash with 200 mL of 1.5 mol / L NaOH solution for several times, and finally wash with deionized water until neutral, remove the chloroform by rotary evaporation, and dry in vacuum at 70 °C. Dry for 5h to obtain the product. FTIR,ν(cm -1 , KBr tablet): 939.42 (characteristic peak of oxazine ring), 750.34 and 828.05 (C-H bending vibratio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tensile shear strength | aaaaa | aaaaa |
| Tensile shear strength | aaaaa | aaaaa |
| Thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com