Test paper applied to detection of coxiella burnetii antibody, preparation method of test paper and kit
A technology for detecting Coxie bodies and test strips, which is applied to measuring devices, instruments, scientific instruments, etc., can solve the problems of low effective antigen content, reduced sensitivity, and reduced amount of labeled antibodies, so as to achieve accurate, reliable and safe detection results Sexual problems improve, amplify the effect of the response effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
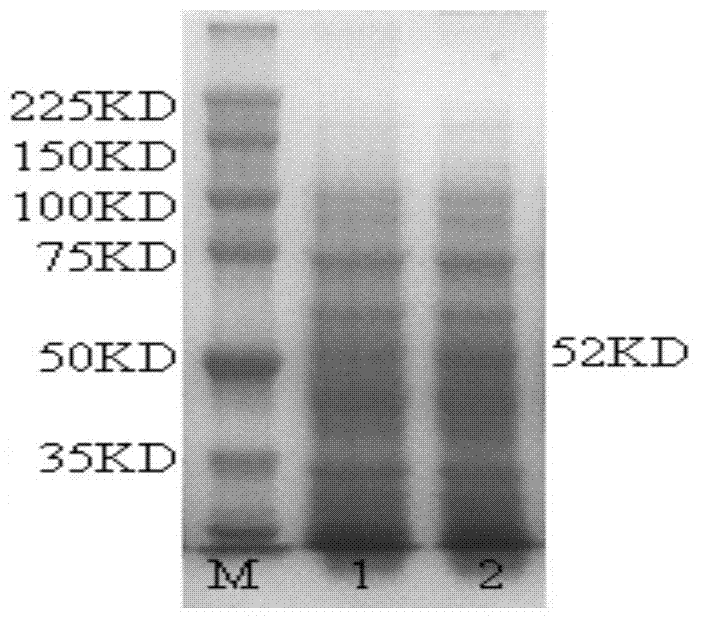
[0047] Example 1 Preparation of Coxiella bezieri antigen
[0048] Using recombinant proteins to prepare antigens of corresponding pathogens is one of the ways to effectively solve the safety problem of pathogens and improve the detection rate of pathogens. At present, there is no report of a recombinant protein that can effectively serve as an antigen of Coxiella bezieri.
[0049] The specific steps for preparing the Coxiella bezieri antigen are as follows:
[0050] a. Construction of recombinant plasmids
[0051] For the selection of the Coxiella beinii antigen, the inventors of the present invention conducted multiple tests, and finally selected the Coxiella beinii envelope P1 protein, whose amino acid sequence reference (GenBank sequence number: AY249911.1) was based on Escherichia coli Escherichia coli O127:H6 preferred codons were used to modify the gene sequence, and the secondary structure of the recombinant protein was screened through bioinformatics (see the referen...
Embodiment 2
[0058] Example 2 Preparation of test strips for the detection of Coxiella basilica antibodies
[0059] A. SPA labeling magnetic nanoparticles, and preparing magnetic nanoparticle carrier pads:
[0060] Take 100 μl ferroferric oxide magnetic nanoparticle solution (the particle size of ferric oxide tetroxide is preferably 12nm), add 700 μl water, 200 μl 25% glutaraldehyde solution, shake for 3 h; wash 1-2 times with 1 ml water, and wash 2 times in PBS The second time is to carry out carboxylation treatment on ferric ferric oxide magnetic nanoparticles; add 500 μl of 2 mg / ml SPA, shake for 3 hours, and the pH value is 7.6; add 500 μl of 0.5% BSA, shake for 30 minutes, and block; add 0.01% spit Warm-20 PBS with a concentration of 0.01M, wash 3-4 times; add 1mL resuspension solution, wherein the resuspension solution contains 0.1% Tris base, 1% BSA and 5% sucrose by weight percentage, mix well, Spray on glass fiber membrane and dry at 37°C for 4 hours;
[0061] B. Coated nitrocel...
Embodiment 3
[0066]Example 3 The detection test paper of Coxella basidiobacillus antibody prepared by the present invention and the detection of the sensitivity of colloidal gold immunochromatography test paper
[0067] Wherein, see embodiment 2 for the preparation method of the detection test paper of the Coxiella bezii antibody prepared by the present invention;
[0068] The preparation method of colloidal gold immunochromatography test paper is as follows:
[0069] Colloidal gold immunochromatography test paper was prepared by conventional methods, wherein SPA-labeled colloidal gold particles were used, the detection zone on the nitrocellulose membrane was coated with Cox's body recombinant antigen, and the quality control zone was coated with goat anti-rabbit IgG.
[0070] The test strips prepared in Example 2 were used to detect the Coxiella bezieri antibody serum respectively, diluted with fetal bovine serum to 1:10, 1:100, 1:1000, 1:10000, 1:100000, 1:1000000, At the same time, tak...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com