Fluoroquinolone substitute template molecularly imprinted polymer and application thereof
A technology of fluoroquinolones and substitute templates, applied in ion exchange, other chemical processes, ion exchange regeneration, etc., to achieve high specific selectivity, ultra-high specific selectivity, and reduce matrix interference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
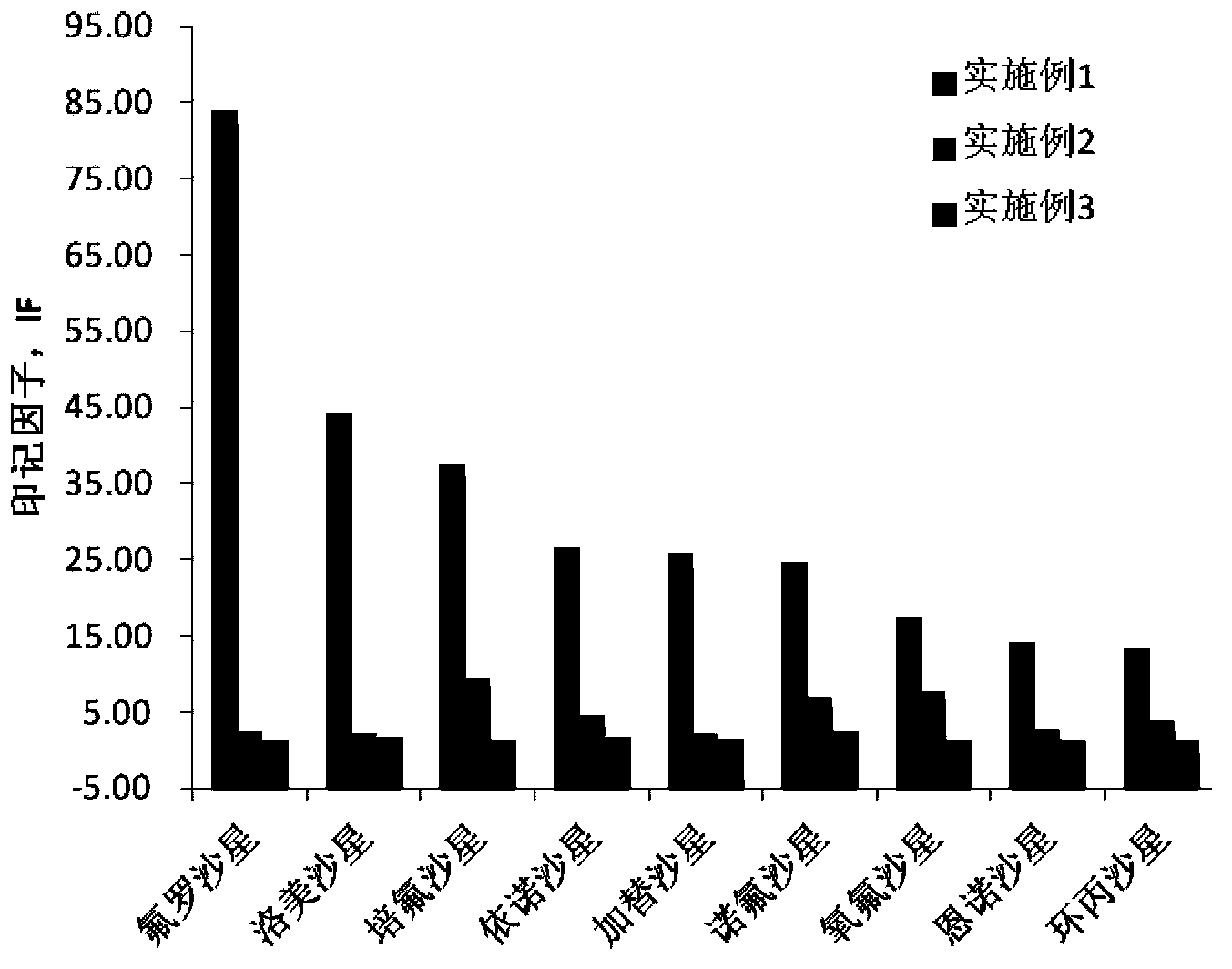
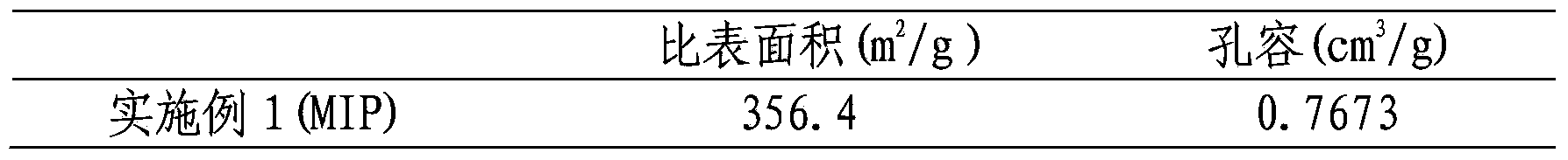
Embodiment 1
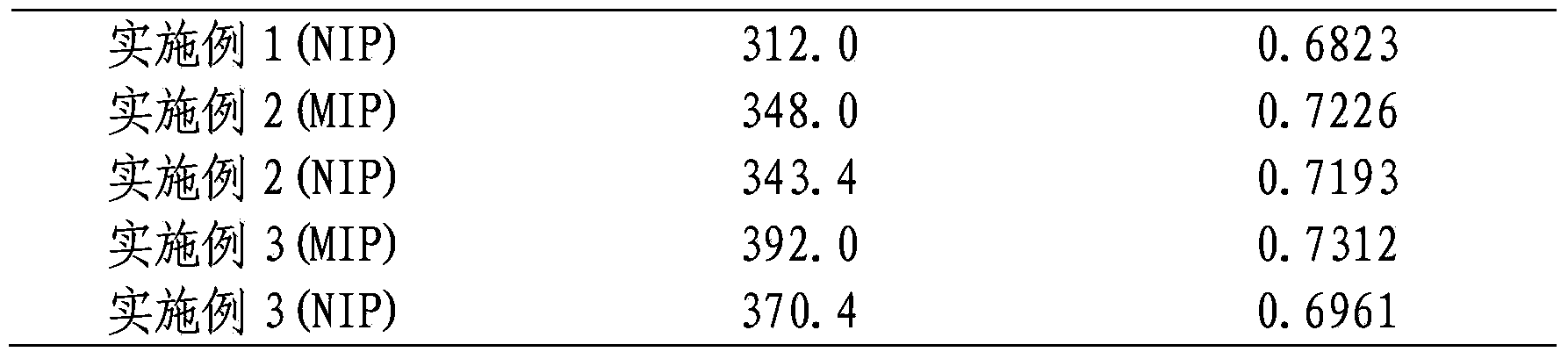
[0022] Dissolve 1 mmol of the surrogate template molecule daidzein into dimethyl dimethacrylate containing 4 mmol of the functional monomer 4-vinylpyridine, 20 mmol of the crosslinker ethylene glycol dimethacrylate and 0.04 g of the initiator azobisisobutyronitrile Prepare a pre-polymerization solution in sulfoxide + acetonitrile (1+2) (5.6mL), place the pre-polymerization solution in an ice bath for 10-15min and mix it with ultrasound, then pass nitrogen for 10min to remove the oxygen in the system, seal it and put it in 4 ℃ for 2 hours, and then placed at 60 ℃ for 24 hours. The white massive polymer produced by the reaction is pulverized, ground, sieved and settled to obtain a polymer with a particle size of 45-63 μm. The obtained polymer was sequentially Soxhlet-extracted using methanol acetic acid (9:1, v / v) and methanol as extraction solvents to remove template molecules and interfering substances, and the obtained polymer was dried overnight in a vacuum oven at 60°C A w...
Embodiment 2
[0029]Dissolve 1 mmol of the surrogate template molecule daidzein into dimethyl dimethacrylate containing 4 mmol of the functional monomer 4-vinylpyridine, 20 mmol of the crosslinker ethylene glycol dimethacrylate and 0.04 g of the initiator azobisisobutyronitrile Prepare a pre-polymerization solution in sulfoxide (5.6mL), place the pre-polymerization solution in an ice bath for 10-15min and mix it with ultrasound, then pass nitrogen gas for 10min to remove the oxygen in the system, seal it and place it in a refrigerator at 4°C for 2h, then Placed at 60°C for 24h. The white massive polymer produced by the reaction is pulverized, ground, sieved and settled to obtain a polymer with a particle size of 45-63 μm. The obtained polymer was sequentially Soxhlet-extracted using methanol acetic acid (9:1, v / v) and methanol as the extraction solvent to remove template molecules and interfering substances, and the obtained polymer was dried overnight in a vacuum oven at 60°C A white mole...
Embodiment 3
[0032] Dissolve 1 mmol of the surrogate template molecule daidzein into dimethyl dimethacrylate containing 4 mmol of the functional monomer 4-vinylpyridine, 20 mmol of the crosslinker ethylene glycol dimethacrylate and 0.04 g of the initiator azobisisobutyronitrile Prepare a pre-polymerization solution in formamide + acetonitrile (1+2), place the pre-polymerization solution in an ice bath for 10-15 minutes and mix it with ultrasound, then pass nitrogen for 10 minutes to remove oxygen in the system, seal it and store it in refrigeration at 4°C 2h, and then placed at 60°C for 24h. The white massive polymer produced by the reaction is pulverized, ground, sieved and settled to obtain a polymer with a particle size of 45-63 μm. The obtained polymer was subjected to Soxhlet extraction using methanol / acetic acid (9:1, v / v) and methanol as extraction solvents in order to remove template molecules and interfering substances, and the obtained polymer was dried in a vacuum oven at 60°C ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com