A coating process for drug coating on implanted or interventional medical devices
A medical device and drug coating technology, applied in coatings, medical science, medical containers, etc., can solve problems such as blood vessel blockage and safety concerns, and achieve the effect of avoiding blood vessel blockage, thrombus formation, and small coating shedding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1: Preparation method of paclitaxel drug-coated balloon
[0040] 1) Preparation of spraying solution: Weigh about 0.15g of paclitaxel and 0.3g of PVP into a 25ml glass bottle; add 10ml of acetonitrile into the glass bottle; keep warm in an oven at 45°C until paclitaxel and PVP are completely dissolved.
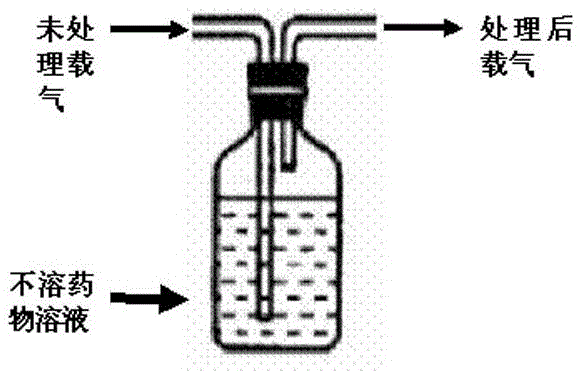
[0041]2) Preparation of carrier gas: pass dry high-purity nitrogen through 95% (volume) ethanol / water mixed solution, install a 0.25μm filter membrane at the outlet of the pipeline, and filter fine droplets;
[0042] 3) Balloon coating: place a 4.0mm*60mm balloon under the ultrasonic spray head, set the ultrasonic frequency to 30khz, the flow rate of the spray solution to 0.1ml / min, the rotation speed of the balloon to 5r / s, and the spraying time For 2min, the carrier gas flow rate is: 30L / h. After spraying, remove the balloon.
[0043] 4) The balloon was dried at room temperature for 30 minutes.
[0044] 5) Fold the balloon, put it into the coil, pack and...
Embodiment 2
[0046] Embodiment 2: Preparation method of rapamycin drug-coated balloon
[0047] 1) Preparation of spraying solution: weigh about 0.13g rapamycin and 0.3g PVA, add to a 25ml glass bottle; add 10ml of ethanol to the glass bottle, stir to dissolve the drug completely.
[0048] 2) Preparation of carrier gas: pass dry air through 75% (volume) acetonitrile / water mixed solvent, and install a 0.25 μm filter membrane at the outlet of the pipeline to filter fine droplets;
[0049] 3) Balloon coating: place a 4.0mm*60mm balloon under the ultrasonic spray head, set the ultrasonic frequency to 50khz, the flow rate of the spray solution to 0.2ml / min, the rotation speed of the balloon to 10r / s, and the spraying time 1.5min, carrier gas flow rate: 30L / h. After spraying, remove the balloon.
[0050] 4) The balloon was dried at room temperature for 30 minutes.
[0051] 5) Fold the balloon, put it into the coil, pack and sterilize.
Embodiment 3
[0052] Embodiment 3: Preparation method of rapamycin drug-coated balloon
[0053] 1) Preparation of spraying solution: Weigh about 0.25g rapamycin and 0.25g PEG into a 25ml glass bottle; add 10ml methanol into the glass bottle and stir to dissolve the drug completely.
[0054] 2) Preparation of carrier gas: pass dry oxygen through water for injection, and install a 0.25μm filter membrane at the outlet of the pipeline to filter fine droplets;
[0055] 3) Balloon coating: place a 4.0mm*60mm balloon under the ultrasonic spray head, set the ultrasonic frequency to 45khz, the flow rate of the spray solution to 0.18ml / min, the balloon rotation speed to 4r / s, and the spraying time 1.2min, carrier gas flow rate: 25L / h. After spraying, remove the balloon.
[0056] 4) The balloon is vacuum dried at 30°C for 20 minutes.
[0057] 5) Fold the balloon, put it into the coil, pack and sterilize.
[0058] Balloon coating performance test:
[0059] 1. Test method and result of drug coating...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com